Phase 1a dose escalation study of ivonescimab (AK112/SMT112), an anti-PD-1/VEGF-A bispecific antibody, in patients with advanced solid tumors
- PMID: 38642937
- PMCID: PMC11033648
- DOI: 10.1136/jitc-2023-008037
Phase 1a dose escalation study of ivonescimab (AK112/SMT112), an anti-PD-1/VEGF-A bispecific antibody, in patients with advanced solid tumors
Abstract
Background: Studies showed that vascular endothelial growth factor (VEGF) inhibitors could improve therapeutic efficacy of PD-1/PD-L1 antibodies by transforming the immunosuppressive tumor microenvironment (TME) into an immunoresponsive TME. Ivonescimab is a first-in-class, humanized tetravalent bispecific antibody targeting PD-1 and VEGF-A simultaneously. Here, we report the first-in-human, phase 1a study of ivonescimab in patients with advanced solid tumors.
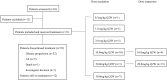
Methods: Patients with advanced solid tumors were treated with ivonescimab 0.3, 1, 3, 10, 20 or 30 mg/kg intravenously every 2 weeks using a 3+3+3 dose escalation design. Dose expansion occurred at 10 and 20 mg/kg in selected tumor types. The primary objective was to assess the safety and tolerability, and to determine the maximum tolerated dose (MTD). The secondary objectives included pharmacokinetics, pharmacodynamics and preliminary antitumor activity based on Response Evaluation Criteria in Solid Tumors V.1.1.
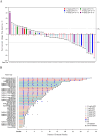
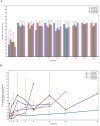
Results: Between October 2, 2019 and January 14, 2021, a total of 51 patients were enrolled and received ivonescimab. Two dose-limiting toxicities were reported at 30 mg/kg. The MTD of ivonescimab was 20 mg/kg every 2 weeks. Grade≥3 treatment-related adverse events (TRAEs) occurred in 14 patients (27.5%). The most common TRAEs of any grade were rash (29.4%), arthralgia (19.6%), hypertension (19.6%), fatigue (17.6%), diarrhea (15.7%) and pruritus (11.8%). The most common grade≥3 TRAEs were hypertension (7/51, 13.7%), alanine aminotransferase increased (3/51, 5.2%), aspartate aminotransferase increased (2/51, 3.9%) and colitis (2/51, 3.9%). Of 47 patients who had at least one postbaseline assessment, the confirmed objective response rate was 25.5% (12/47) and disease control rate was 63.8% (30/47). Among 19 patients with platinum-resistant ovarian cancer, 5 patients (26.3%) achieved partial response (PR). Efficacy signals were also observed in patients with mismatch repair proficient (pMMR) colorectal cancer, non-small cell lung cancer, and both MMR deficient and pMMR endometrial cancer.
Conclusions: Ivonescimab demonstrated manageable safety profiles and promising efficacy signals in multiple solid tumors. Exploration of alternative dosing regimens of ivonescimab monotherapy and combination therapies is warranted.
Trial registration number: NCT04047290.
Keywords: antibodies, neoplasm; clinical trials as topic.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: WW, ZMW, BL, and YX are all employees of Akeso Biopharma, Zhongshan, China. JIGC provided consultancy work for Akeso since 2022. The other authors declare no potential conflicts of interest.
Figures

References
-
- Gabrilovich D, Ishida T, Oyama T, et al. . Vascular endothelial growth factor inhibits the development of Dendritic cells and dramatically affects the differentiation of multiple hematopoietic lineages in vivo. Blood 1998;92:4150–66. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
