Sleep and circadian biomarkers of postoperative delirium (SLEEP-POD): protocol for a prospective and observational cohort study
- PMID: 38643014
- PMCID: PMC11033637
- DOI: 10.1136/bmjopen-2023-080796
Sleep and circadian biomarkers of postoperative delirium (SLEEP-POD): protocol for a prospective and observational cohort study
Abstract
Introduction: Surgical patients over 70 experience postoperative delirium (POD) complications in up to 50% of procedures. Sleep/circadian disruption has emerged as a potential risk factor for POD in epidemiological studies. This protocol presents a single-site, prospective observational study designed to examine the relationship between sleep/circadian regulation and POD and how this association could be moderated or mediated by Alzheimer's disease (AD) pathology and genetic risk for AD.
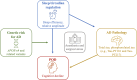
Methods and analysis: Study staff members will screen for eligible patients (age ≥70) seeking joint replacement or spinal surgery at Massachusetts General Hospital (MGH). At the inclusion visit, patients will be asked a series of questionnaires related to sleep and cognition, conduct a four-lead ECG recording and be fitted for an actigraphy watch to wear for 7 days before surgery. Blood samples will be collected preoperatively and postoperatively and will be used to gather information about AD variant genes (APOE-ε4) and AD-related pathology (total and phosphorylated tau). Confusion Assessment Method-Scale and Montreal Cognitive Assessment will be completed twice daily for 3 days after surgery. Seven-day actigraphy assessments and Patient-Reported Outcomes Measurement Information System questionnaires will be performed 1, 3 and 12 months after surgery. Relevant patient clinical data will be monitored and recorded throughout the study.
Ethics and dissemination: This study is approved by the IRB at MGH, Boston, and it is registered with the US National Institutes of Health on ClinicalTrials.gov (NCT06052397). Plans for dissemination include conference presentations at a variety of scientific institutions. Results from this study are intended to be published in peer-reviewed journals. Relevant updates will be made available on ClinicalTrials.gov.
Trial registration number: NCT06052397.
Keywords: Anaesthesia in neurology; Delirium & cognitive disorders; Dementia; GENETICS; Sleep medicine.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: ZX provided consulting services to Baxter Pharmaceutical company, Shanghai 9th and 10th Hospital, NanoMosaic, and Anesthesiology and Perioperative Science within the last 36 months. The authors have no other conflicts of interest to declare.
Figures
References
-
- American Geriatrics Society Expert Panel on Postoperative Delirium in Older Adults . Postoperative delirium in older adults: best practice statement from the American Geriatrics society. J Am Coll Surg 2015;220:136–48:e1. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous