Dimorphic effect of TFE3 in determining mitochondrial and lysosomal content in muscle following denervation
- PMID: 38643162
- PMCID: PMC11031958
- DOI: 10.1186/s13395-024-00339-1
Dimorphic effect of TFE3 in determining mitochondrial and lysosomal content in muscle following denervation
Abstract
Background: Muscle atrophy is a common consequence of the loss of innervation and is accompanied by mitochondrial dysfunction. Mitophagy is the adaptive process through which damaged mitochondria are removed via the lysosomes, which are regulated in part by the transcription factor TFE3. The role of lysosomes and TFE3 are poorly understood in muscle atrophy, and the effect of biological sex is widely underreported.
Methods: Wild-type (WT) mice, along with mice lacking TFE3 (KO), a transcriptional regulator of lysosomal and autophagy-related genes, were subjected to unilateral sciatic nerve denervation for up to 7 days, while the contralateral limb was sham-operated and served as an internal control. A subset of animals was treated with colchicine to capture mitophagy flux.
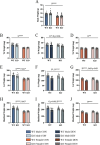
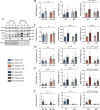
Results: WT females exhibited elevated oxygen consumption rates during active respiratory states compared to males, however this was blunted in the absence of TFE3. Females exhibited higher mitophagy flux rates and greater lysosomal content basally compared to males that was independent of TFE3 expression. Following denervation, female mice exhibited less muscle atrophy compared to male counterparts. Intriguingly, this sex-dependent muscle sparing was lost in the absence of TFE3. Denervation resulted in 45% and 27% losses of mitochondrial content in WT and KO males respectively, however females were completely protected against this decline. Decreases in mitochondrial function were more severe in WT females compared to males following denervation, as ROS emission was 2.4-fold higher. In response to denervation, LC3-II mitophagy flux was reduced by 44% in females, likely contributing to the maintenance of mitochondrial content and elevated ROS emission, however this response was dysregulated in the absence of TFE3. While both males and females exhibited increased lysosomal content following denervation, this response was augmented in females in a TFE3-dependent manner.
Conclusions: Females have higher lysosomal content and mitophagy flux basally compared to males, likely contributing to the improved mitochondrial phenotype. Denervation-induced mitochondrial adaptations were sexually dimorphic, as females preferentially preserve content at the expense of function, while males display a tendency to maintain mitochondrial function. Our data illustrate that TFE3 is vital for the sex-dependent differences in mitochondrial function, and in determining the denervation-induced atrophy phenotype.
Keywords: Autophagy; Lysosomal biogenesis; Mitochondrial respiration; Mitophagy; ROS emission; Sex differences.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Memme JM, Slavin M, Moradi N, Hood DA. Mitochondrial Bioenergetics and Turnover during Chronic Muscle Disuse. Int J Mol Sci. 2021, Vol 22, Page 5179 [Internet]. Multidisciplinary Digital Publishing Institute; 2021 [cited 2022 Jan 10];22:5179. Available from: https://www.mdpi.com/1422-0067/22/10/5179/htm. - PMC - PubMed
-
- Powers SK, Wiggs MP, Duarte JA, Murat Zergeroglu A, Demirel HA, Zergeroglu AM et al. Mitochondrial signaling contributes to disuse muscle atrophy. Am J Physiol Endocrinol Metab [Internet]. 2012 [cited 2018 Feb 27];303:E31–9. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3404565%7B&%7D... - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

