Phosphoproteomic analysis reveals changes in A-Raf-related protein phosphorylation in response to Toxoplasma gondii infection in porcine macrophages
- PMID: 38643189
- PMCID: PMC11031963
- DOI: 10.1186/s13071-024-06273-x
Phosphoproteomic analysis reveals changes in A-Raf-related protein phosphorylation in response to Toxoplasma gondii infection in porcine macrophages
Abstract
Background: Toxoplasma gondii is an obligate intracellular protozoan parasite that causes severe threats to humans and livestock. Macrophages are the cell type preferentially infected by T. gondii in vivo. Protein phosphorylation is an important posttranslational modification involved in diverse cellular functions. A rapidly accelerated fibrosarcoma kinase (A-Raf) is a member of the Raf family of serine/threonine protein kinases that is necessary for MAPK activation. Our previous research found that knockout of A-Raf could reduce T. gondii-induced apoptosis in porcine alveolar macrophages (3D4/21 cells). However, limited information is available on protein phosphorylation variations and the role of A-Raf in macrophages infected with T. gondii.
Methods: We used immobilized metal affinity chromatography (IMAC) in combination with liquid chromatography tandem mass spectrometry (LC-MS/MS) to profile changes in phosphorylation in T. gondii-infected 3D4/21 and 3D4/21-ΔAraf cells.
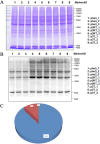
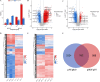
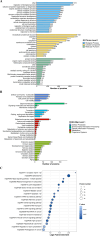
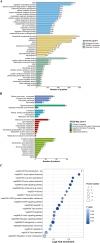
Results: A total of 1647 differentially expressed phosphorylated proteins (DEPPs) with 3876 differentially phosphorylated sites (DPSs) were identified in T. gondii-infected 3D4/21 cells (p3T group) when compared with uninfected 3D4/21 cells (pho3 group), and 959 DEPPs with 1540 DPSs were identified in the p3T group compared with infected 3D4/21-ΔAraf cells (p3KT group). Venn analysis revealed 552 DPSs corresponding to 406 DEPPs with the same phosphorylated sites when comparing p3T/pho3 versus p3T/p3KT, which were identified as DPSs and DEPPs that were directly or indirectly related to A-Raf.
Conclusions: Our results revealed distinct responses of macrophages to T. gondii infection and the potential roles of A-Raf in fighting infection via phosphorylation of crucial proteins.
Keywords: Toxoplasma gondii; 3D4/21; A-Raf; Apoptosis; Host cells; Phosphorylation.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


Similar articles
-
Expression profile of microRNAs in porcine alveolar macrophages after Toxoplasma gondii infection.Parasit Vectors. 2019 Jan 29;12(1):65. doi: 10.1186/s13071-019-3297-y. Parasit Vectors. 2019. PMID: 30696482 Free PMC article.
-
Toxoplasma gondii infection regulates apoptosis of host cells via miR-185/ARAF axis.Parasit Vectors. 2023 Oct 19;16(1):371. doi: 10.1186/s13071-023-05991-y. Parasit Vectors. 2023. PMID: 37858158 Free PMC article.
-
Brain proteomic differences between wild-type and CD44- mice induced by chronic Toxoplasma gondii infection.Parasitol Res. 2018 Aug;117(8):2623-2633. doi: 10.1007/s00436-018-5954-z. Epub 2018 Jun 12. Parasitol Res. 2018. PMID: 29948204
-
Apoptosis and its modulation during infection with Toxoplasma gondii: molecular mechanisms and role in pathogenesis.Curr Top Microbiol Immunol. 2005;289:219-37. doi: 10.1007/3-540-27320-4_10. Curr Top Microbiol Immunol. 2005. PMID: 15791958 Review.
-
Molecular modifications of host cells by Toxoplasma gondii.Pol J Microbiol. 2004;53 Suppl:45-54. Pol J Microbiol. 2004. PMID: 15787197 Review.
References
MeSH terms
Grants and funding
- 32373041/National Natural Science Foundation of China
- 2020M671615/China Postdoctoral Science Foundation
- 6/International Research Laboratory of Prevention and Control of Important Animal Infectious Diseases and Zoonotic Diseases of Jiangsu Higher Education Institutions
- BK20190885/Basic Research Program of Jiangsu Province
- RCPY201929/Taizhou Project of Scientific Research for Talent Cultivation
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

