Replicative senescence and high glucose induce the accrual of self-derived cytosolic nucleic acids in human endothelial cells
- PMID: 38643201
- PMCID: PMC11032409
- DOI: 10.1038/s41420-024-01954-z
Replicative senescence and high glucose induce the accrual of self-derived cytosolic nucleic acids in human endothelial cells
Abstract
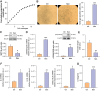
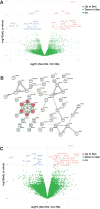
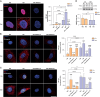
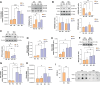
Recent literature shows that loss of replicative ability and acquisition of a proinflammatory secretory phenotype in senescent cells is coupled with the build-in of nucleic acids in the cytoplasm. Its implication in human age-related diseases is under scrutiny. In human endothelial cells (ECs), we assessed the accumulation of intracellular nucleic acids during in vitro replicative senescence and after exposure to high glucose concentrations, which mimic an in vivo condition of hyperglycemia. We showed that exposure to high glucose induces senescent-like features in ECs, including telomere shortening and proinflammatory cytokine release, coupled with the accrual in the cytoplasm of telomeres, double-stranded DNA and RNA (dsDNA, dsRNA), as well as RNA:DNA hybrid molecules. Senescent ECs showed an activation of the dsRNA sensors RIG-I and MDA5 and of the DNA sensor TLR9, which was not paralleled by the involvement of the canonical (cGAS) and non-canonical (IFI16) activation of the STING pathway. Under high glucose conditions, only a sustained activation of TLR9 was observed. Notably, senescent cells exhibit increased proinflammatory cytokine (IL-1β, IL-6, IL-8) production without a detectable secretion of type I interferon (IFN), a phenomenon that can be explained, at least in part, by the accumulation of methyl-adenosine containing RNAs. At variance, exposure to exogenous nucleic acids enhances both IL-6 and IFN-β1 expression in senescent cells. This study highlights the accrual of cytoplasmic nucleic acids as a marker of senescence-related endothelial dysfunction, that may play a role in dysmetabolic age-related diseases.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

