Regulation of enzymatic reactions by chemical composition of peptide biomolecular condensates
- PMID: 38643237
- PMCID: PMC11032315
- DOI: 10.1038/s42004-024-01174-7
Regulation of enzymatic reactions by chemical composition of peptide biomolecular condensates
Abstract
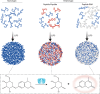
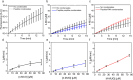
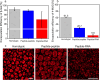
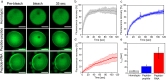
Biomolecular condensates are condensed intracellular phases that are formed by liquid-liquid phase separation (LLPS) of proteins, either in the absence or presence of nucleic acids. These condensed phases regulate various biochemical reactions by recruitment of enzymes and substrates. Developments in the field of LLPS facilitated new insights on the regulation of compartmentalized enzymatic reactions. Yet, the influence of condensate chemical composition on enzymatic reactions is still poorly understood. Here, by using peptides as minimalistic condensate building blocks and β-galactosidase as a simple enzymatic model we show that the reaction is restricted in homotypic peptide condensates, while product formation is enhanced in peptide-RNA condensates. Our findings also show that condensate composition affects the recruitment of substrate, the spatial distribution, and the kinetics of the reaction. Thus, these findings can be further employed for the development of microreactors for biotechnological applications.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interest.
Figures

Similar articles
-
Coacervates as enzymatic microreactors.Chem Soc Rev. 2025 May 6;54(9):4183-4199. doi: 10.1039/d4cs01203h. Chem Soc Rev. 2025. PMID: 40084439 Free PMC article. Review.
-
Regulation of Peptide Liquid-Liquid Phase Separation by Aromatic Amino Acid Composition.Small. 2024 Sep;20(38):e2401665. doi: 10.1002/smll.202401665. Epub 2024 May 28. Small. 2024. PMID: 38804888
-
Biomolecular condensates formed by designer minimalistic peptides.Nat Commun. 2023 Jan 26;14(1):421. doi: 10.1038/s41467-023-36060-8. Nat Commun. 2023. PMID: 36702825 Free PMC article.
-
Biomolecular Condensates Regulate Enzymatic Activity under a Crowded Milieu: Synchronization of Liquid-Liquid Phase Separation and Enzymatic Transformation.J Phys Chem B. 2023 Jan 12;127(1):180-193. doi: 10.1021/acs.jpcb.2c07684. Epub 2023 Jan 3. J Phys Chem B. 2023. PMID: 36594499
-
Higher-order organization of biomolecular condensates.Open Biol. 2021 Jun;11(6):210137. doi: 10.1098/rsob.210137. Epub 2021 Jun 16. Open Biol. 2021. PMID: 34129784 Free PMC article. Review.
Cited by
-
Coacervation in systems chemistry.Commun Chem. 2024 Nov 22;7(1):275. doi: 10.1038/s42004-024-01358-1. Commun Chem. 2024. PMID: 39578544 Free PMC article.
-
Structured protein domains enter the spotlight: modulators of biomolecular condensate form and function.Trends Biochem Sci. 2025 Mar;50(3):206-223. doi: 10.1016/j.tibs.2024.12.008. Epub 2025 Jan 17. Trends Biochem Sci. 2025. PMID: 39827079 Review.
-
Coacervates as enzymatic microreactors.Chem Soc Rev. 2025 May 6;54(9):4183-4199. doi: 10.1039/d4cs01203h. Chem Soc Rev. 2025. PMID: 40084439 Free PMC article. Review.
-
Tailoring Peptide Coacervates for Advanced Biotechnological Applications: Enhancing Control, Encapsulation, and Antioxidant Properties.ACS Appl Mater Interfaces. 2025 May 28;17(21):31561-31574. doi: 10.1021/acsami.5c02367. Epub 2025 Apr 28. ACS Appl Mater Interfaces. 2025. PMID: 40296204 Free PMC article.
-
Recombinase-Controlled Multiphase Condensates Accelerate Nucleic Acid Amplification and CRISPR-Based Diagnostics.J Am Chem Soc. 2025 Mar 26;147(12):10088-10103. doi: 10.1021/jacs.4c11893. Epub 2025 Feb 13. J Am Chem Soc. 2025. PMID: 39948709 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources

