A practical synthesis of nitrone-derived C5a-functionalized isofagomines as protein stabilizers to treat Gaucher disease
- PMID: 38643239
- PMCID: PMC11032326
- DOI: 10.1038/s42004-024-01164-9
A practical synthesis of nitrone-derived C5a-functionalized isofagomines as protein stabilizers to treat Gaucher disease
Abstract
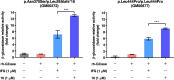
Isofagomine (IFG) and its analogues possess promising glycosidase inhibitory activities. However, a flexible synthetic strategy toward both C5a-functionalized IFGs remains to be explored. Here we show a practical synthesis of C5a-S and R aminomethyl IFG-based derivatives via the diastereoselective addition of cyanide to cyclic nitrone 1. Nitrone 1 was conveniently prepared on a gram scale and in high yield from inexpensive (-)-diethyl D-tartrate via a straightforward method, with a stereoselective Michael addition of a nitroolefin and a Nef reaction as key steps. A 268-membered library (134 × 2) of the C5a-functionalized derivatives was submitted to enzyme- or cell-based bio-evaluations, which resulted in the identification of a promising β-glucocerebrosidase (GCase) stabilizer demonstrating a 2.7-fold enhancement at 25 nM in p.Asn370Ser GCase activity and a 13-fold increase at 1 μM in recombinant human GCase activity in Gaucher cell lines.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
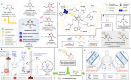
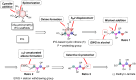
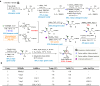

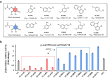
References
-
- Thiel P, Kaiser M, Ottmann C. Small-molecule stabilization of protein–protein interactions: an underestimated concept in drug discovery? Angew. Chem. Int. Ed. 2012;51:2012–2018. - PubMed
-
- Bernier V, Lagacé M, Bichet DG, Bouvier M. Pharmacological chaperones: potential treatment for conformational diseases. Trends Endocrinol. Metab. 2004;15:222–228. - PubMed
-
- Bernier V, et al. Pharmacologic chaperones as a potential treatment for X-linked nephrogenic diabetes insipidus. J. Am. Soc. Nephrol. 2006;17:232–243. - PubMed
LinkOut - more resources
Full Text Sources

