8-Cl-Ado and 8-NH2-Ado synergize with venetoclax to target the methionine-MAT2A-SAM axis in acute myeloid leukemia
- PMID: 38643304
- PMCID: PMC11147765
- DOI: 10.1038/s41375-024-02222-w
8-Cl-Ado and 8-NH2-Ado synergize with venetoclax to target the methionine-MAT2A-SAM axis in acute myeloid leukemia
Abstract
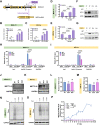
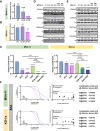
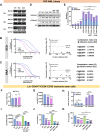
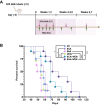
Targeting the metabolic dependencies of acute myeloid leukemia (AML) cells is a promising therapeutical strategy. In particular, the cysteine and methionine metabolism pathway (C/M) is significantly altered in AML cells compared to healthy blood cells. Moreover, methionine has been identified as one of the dominant amino acid dependencies of AML cells. Through RNA-seq, we found that the two nucleoside analogs 8-chloro-adenosine (8CA) and 8-amino-adenosine (8AA) significantly suppress the C/M pathway in AML cells, and methionine-adenosyltransferase-2A (MAT2A) is one of most significantly downregulated genes. Additionally, mass spectrometry analysis revealed that Venetoclax (VEN), a BCL-2 inhibitor recently approved by the FDA for AML treatment, significantly decreases the intracellular level of methionine in AML cells. Based on these findings, we hypothesized that combining 8CA or 8AA with VEN can efficiently target the Methionine-MAT2A-S-adenosyl-methionine (SAM) axis in AML. Our results demonstrate that VEN and 8CA/8AA synergistically decrease the SAM biosynthesis and effectively target AML cells both in vivo and in vitro. These findings suggest the promising potential of combining 8CA/8AA and VEN for AML treatment by inhibiting Methionine-MAT2A-SAM axis and provide a strong rationale for our recently activated clinical trial.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin. 2021;71:7–33. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

