A phase 3 randomized trial of mavorixafor, a CXCR4 antagonist, for WHIM syndrome
- PMID: 38643510
- PMCID: PMC11251404
- DOI: 10.1182/blood.2023022658
A phase 3 randomized trial of mavorixafor, a CXCR4 antagonist, for WHIM syndrome
Abstract
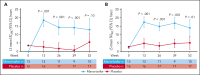
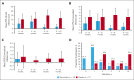
We investigated efficacy and safety of mavorixafor, an oral CXCR4 antagonist, in participants with warts, hypogammaglobulinemia, infections, and myelokathexis (WHIM) syndrome, a rare immunodeficiency caused by CXCR4 gain-of-function variants. This randomized (1:1), double-blind, placebo-controlled, phase 3 trial enrolled participants aged ≥12 years with WHIM syndrome and absolute neutrophil count (ANC) ≤0.4 × 103/μL. Participants received once-daily mavorixafor or placebo for 52 weeks. The primary end point was time (hours) above ANC threshold ≥0.5 × 103/μL (TATANC; over 24 hours). Secondary end points included TAT absolute lymphocyte count ≥1.0 × 103/μL (TATALC; over 24 hours); absolute changes in white blood cell (WBC), ANC, and absolute lymphocyte count (ALC) from baseline; annualized infection rate; infection duration; and total infection score (combined infection number/severity). In 31 participants (mavorixafor, n = 14; placebo, n = 17), mavorixafor least squares (LS) mean TATANC was 15.0 hours and 2.8 hours for placebo (P < .001). Mavorixafor LS mean TATALC was 15.8 hours and 4.6 hours for placebo (P < .001). Annualized infection rates were 60% lower with mavorixafor vs placebo (LS mean 1.7 vs 4.2; nominal P = .007), and total infection scores were 40% lower (7.4 [95% confidence interval [CI], 1.6-13.2] vs 12.3 [95% CI, 7.2-17.3]). Treatment with mavorixafor reduced infection frequency, severity, duration, and antibiotic use. No discontinuations occurred due to treatment-emergent adverse events (TEAEs); no related serious TEAEs were observed. Overall, mavorixafor treatment demonstrated significant increases in LS mean TATANC and TATALC, reduced infection frequency, severity/duration, and was well tolerated. The trial was registered at www.clinicaltrials.gov as #NCT03995108.
© 2024 American Society of Hematology. Published by Elsevier Inc. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: R.B. is a consultant for X4 Pharmaceuticals, Inc, Angelini Pharma, and Janssen. L.A. has received research funding (to Institu de Recerca Sant Joan de Déu) from CSL Behring, Pharming, and Grifols and is a speaker for Novartis, Sanofi, Roche, and UCB Pharmaceuticals. A.A. has received research funding/grants from X4 Pharmaceuticals, Inc, Grifols, and Argenx; and is a consultant for Grifols, Argenx, Takeda Pharmaceuticals, Adma Biologics, Inc, and Octapharma. A.A.B. has received research funding from X4 Pharmaceuticals, Inc, and the National Institutes of Health. D.D. has consulted, received research funding from, and received honoraria from X4 Pharmaceuticals, Inc. K.E.D. is on the advisory board of Agios. H.J.K. receives research funding from Amgen and is a member on the board of directors or advisory committees for Amgen, Novartis, GPCR Therapeutics, and Cartexell. A.K. has received research funding (to Pavlov University) from X4 Pharmaceuticals, Inc, Alexion, and Apellis and is a speaker for Novartis, Generium, Sobi, AstraZeneca, and Johnson & Johnson. D.L. is a board member for RCPA. C.L. receives research grants from Emek Center Pediatric Hematology University Hospital. J.P. is on the advisory board of Allergy & Anaphylaxis Australia, Food and Allergy Standards Australia and New Zealand, and National Blood Authority and is the director of the Australasian Society of Clinical Immunology and Allergy (QPIAS). A.S. is a speaker for Sobi, Novartis, and Octapharma. T.K.T. is a consultant for X4 Pharmaceuticals, Inc and also receives research funding from X4 Pharmaceuticals, Inc, AbbVie, Inc, Viela Bio, Horizon, and Chiesi. M.G.V. has received research funding outside the current work from Austrian National Bank, a grant from Pfizer, and honoraria from Gilead, Astro Pharma, and Menarini. J.D. is a consultant for X4 Pharmaceuticals, Inc. A.B., K.C., S.D., Y.H., H.J., S.L., R.M., T.Y., and A.G.T. are current employees and/or have equity ownership in X4 Pharmaceuticals, Inc. M.S. was employed by X4 Pharmaceuticals, Inc, at the time of this work, has equity ownership in X4 Pharmaceuticals, Inc, and is a member of the board of directors of X4 Pharmaceuticals, Inc. G.J.B. is a member of the board of directors of X4 Pharmaceuticals, Inc, and has stock options in the company. The remaining authors declare no competing financial interests.
Figures

Comment in
-
Mavorixafor: a new hope for WHIM syndrome.Blood. 2024 Jul 4;144(1):1-2. doi: 10.1182/blood.2024024942. Blood. 2024. PMID: 38963672 No abstract available.
References
-
- Balabanian K, Lagane B, Pablos JL, et al. WHIM syndromes with different genetic anomalies are accounted for by impaired CXCR4 desensitization to CXCL12. Blood. 2005;105(6):2449–2457. - PubMed
-
- Hernandez PA, Gorlin RJ, Lukens JN, et al. Mutations in the chemokine receptor gene CXCR4 are associated with WHIM syndrome, a combined immunodeficiency disease. Nat Genet. 2003;34(1):70–74. - PubMed
-
- Wetzler M, Talpaz M, Kleinerman ES, et al. A new familial immunodeficiency disorder characterized by severe neutropenia, a defective marrow release mechanism, and hypogammaglobulinemia. Am J Med. 1990;89(5):663–672. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Associated data
LinkOut - more resources
Full Text Sources
Medical

