Evaluation of Ornidazole Tablets Bioequivalence in Chinese Healthy Participants Under Fasted and Fed Conditions Using Pharmacokinetic Parameters
- PMID: 38644462
- PMCID: PMC11315871
- DOI: 10.1007/s40268-024-00457-7
Evaluation of Ornidazole Tablets Bioequivalence in Chinese Healthy Participants Under Fasted and Fed Conditions Using Pharmacokinetic Parameters
Abstract
Background and objective: Ornidazole, the third generation of nitroimidazole derivatives after metronidazole and tinidazole, it exerts both bactericidal and antiprotozoal effects. The purpose of this study was to evaluate the pharmacokinetic and bioequivalence of two ornidazole tablets manufactured by two different manufacturers based on their pharmacokinetic parameters.
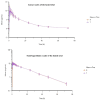
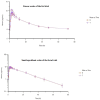
Patients and methods: Fasted and fed healthy Chinese volunteers participated in a randomized sequence, single-dose, open-label, two-period crossover trial. There were 24 participants in both the fed study and the fasted study. Following a 7-day washout period before receiving the alternative formulation, eligible research participants were randomly assigned (1:1) to receive a single dosage of either the reference formulation or the test formulation. Following tablet administration, plasma samples were obtained over 72 h and analyzed using liquid chromatography tandem mass spectrometry (LC-MS/MS) to evaluate ornidazole contents. maximum plasma concentration (Cmax), time to Cmax (Tmax), the area under the curve (AUC) from t = 0 to infinity (AUC0-∞), AUC from t = 0 to the last quantifiable concentration (AUC0-t), half-life (t1/2), and terminal elimination rate constant (z) were evaluated as pharmacokinetic (PK) parameters. The safety evaluation involved adverse events (AEs) incidence and alterations in laboratory tests (hepatic function, blood biochemistry, hematology, and urinalysis) or vital signs (temperature, pulse, and blood pressure).
Results: For the bioequivalence assessment in the fast trial, the prime PK parameters comparison between the reference and test formulation revealed that the GMR (90% CI) values for AUC0-t, Cmax, and AUC0-∞ were 100.97% (99.12-102.85%), 99.88% (90.63-110.08%), and 101.12% (99.17-103.11%), respectively. For the bioequivalence assessment in the fed trial, the key PK parameters comparison between the reference and test formulations revealed that the GMR (90% CI) values for AUC0-t, Cmax, and AUC0-∞ were 103.00% (100.94-105.11%), 101.90% (99.63-104.22%), and 102.99% (100.87-105.16%), respectively. The geometric mean ratios (GMRs) for the primary pharmacokinetic parameters (Cmax, AUC0-72, and AUC0-∞) between the two formulations and the corresponding 90% confidence intervals (CIs) were all within the range of 80.00-125.00% for both the fasting and fed states. Both treatments have comparable safety profiles.
Conclusion: The bioequivalence and tolerability of ornidazole tablet reference and test formulations were evaluated among healthy Chinese participants under both fasting and fed conditions. The results indicated that both formulations were bioequivalent and generally well tolerated; besides, the interaction between food and drug may affect drug pharmacokinetics.
Trial registration: CTR20212873, registered on 15 November 2021; ChiCTR2300069098, registered on 7 March 2023.
© 2024. The Author(s).
Conflict of interest statement
Lijing Gao and Yingzi Pei are affiliated with Beijing Fuyuan Pharmaceutical Co., Ltd. The authors disclose no further conflicts of interest in this study. Zhejiang Aisheng Pharmaceutical Co., Ltd. is a subsidiary of Beijing Fuyuan Pharmaceutical Co. Ltd. This article was related to the 2020 Hebei Province medical science research project (no. 20200340).
Figures
Similar articles
-
Pharmacokinetics and Bioequivalence Evaluation of Two Montelukast Sodium Chewable Tablets in Healthy Chinese Volunteers Under Fasted and Fed Conditions.Drug Des Devel Ther. 2021 Mar 9;15:1091-1099. doi: 10.2147/DDDT.S298355. eCollection 2021. Drug Des Devel Ther. 2021. PMID: 33727797 Free PMC article. Clinical Trial.
-
Pharmacokinetics and Bioequivalence of Two Formulations of Azithromycin Tablets: A Randomized, Single-Dose, Three-Period, Crossover Study in Healthy Chinese Volunteers Under Fasting and Fed Conditions.Drugs R D. 2024 Jun;24(2):201-209. doi: 10.1007/s40268-024-00464-8. Epub 2024 May 30. Drugs R D. 2024. PMID: 38811485 Free PMC article. Clinical Trial.
-
Pharmacokinetics and bioequivalence of Ezetimibe tablet versus Ezetrol®:an open-label, randomized, two-sequence crossover study in healthy Chinese subjects.BMC Pharmacol Toxicol. 2023 Feb 3;24(1):7. doi: 10.1186/s40360-023-00649-y. BMC Pharmacol Toxicol. 2023. PMID: 36737825 Free PMC article. Clinical Trial.
-
Simultaneous confidence regions for multivariate bioequivalence.Stat Med. 2017 Dec 20;36(29):4585-4603. doi: 10.1002/sim.7446. Epub 2017 Aug 30. Stat Med. 2017. PMID: 28857229 Review.
-
Clinical Pharmacokinetics of Fexofenadine: A Systematic Review.Pharmaceutics. 2024 Dec 20;16(12):1619. doi: 10.3390/pharmaceutics16121619. Pharmaceutics. 2024. PMID: 39771597 Free PMC article. Review.
References
-
- http://www.medsafe.govt.nz. Available: July 17, 2014. http://www.medsafe.govt.nz/profs/datasheet/a/arrowornidazoletab.pdf.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

