Transient receptor potential ankyrin 1 channel modulates the abuse-related mechanisms of methamphetamine through interaction with dopamine transporter
- PMID: 38644533
- PMCID: PMC11230846
- DOI: 10.1111/bph.16370
Transient receptor potential ankyrin 1 channel modulates the abuse-related mechanisms of methamphetamine through interaction with dopamine transporter
Abstract
Background and purpose: Methamphetamine (METH) use disorder has risen dramatically over the past decade, and there are currently no FDA-approved medications due, in part, to gaps in our understanding of the pharmacological mechanisms related to METH action in the brain.
Experimental approach: Here, we investigated whether transient receptor potential ankyrin 1 (TRPA1) mediates each of several METH abuse-related behaviours in rodents: self-administration, drug-primed reinstatement, acquisition of conditioned place preference, and hyperlocomotion. Additionally, METH-induced molecular (i.e., neurotransmitter and protein) changes in the brain were compared between wild-type and TRPA1 knock-out mice. Finally, the relationship between TRPA1 and the dopamine transporter was investigated through immunoprecipitation and dopamine reuptake assays.
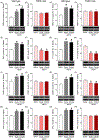
Key results: TRPA1 antagonism blunted METH self-administration and drug-primed reinstatement of METH-seeking behaviour. Further, development of METH-induced conditioned place preference and hyperlocomotion were inhibited by TRPA1 antagonist treatment, effects that were not observed in TRPA1 knock-out mice. Similarly, molecular studies revealed METH-induced increases in dopamine levels and expression of dopamine system-related proteins in wild-type, but not in TRPA1 knock-out mice. Furthermore, pharmacological blockade of TRPA1 receptors reduced the interaction between TRPA1 and the dopamine transporter, thereby increasing dopamine reuptake activity by the transporter.
Conclusion and implications: This study demonstrates that TRPA1 is involved in the abuse-related behavioural effects of METH, potentially through its modulatory role in METH-induced activation of dopaminergic neurotransmission. Taken together, these data suggest that TRPA1 may be a novel therapeutic target for treating METH use disorder.
Keywords: addiction; dopamine transporter; methamphetamine; substance use disorder; transient receptor potential ankyrin 1 channel.
© 2024 The Authors. British Journal of Pharmacology published by John Wiley & Sons Ltd on behalf of British Pharmacological Society.
Conflict of interest statement
Conflict of interest
The authors have no conflicts of interest to declare.
Figures


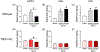


References
-
- Abarca J, Gysling K, Roth RH, & Bustos G (1995). Changes in extracellular levels of glutamate and aspartate in rat substantia nigra induced by dopamine receptor ligands: in vivo microdialysis studies. Neurochem Res, 20, 159–169. - PubMed
-
- Abraini JH, & Rostain J-C (1991). Pressure-induced striatal dopamine release correlates hyperlocomotor activity in rats exposed to high pressure. Journal of Applied Physiology, 71(2), 638–643. - PubMed
-
- Alexander SP, Kelly E, Mathie A, Peters JA, Veale EL, Armstrong JF, … Southan C (2021). The concise guide to pharmacology 2021/22: Transporters. British Journal of Pharmacology, 178, S412–S513. - PubMed
-
- Alexander SP, Mathie A, Peters JA, Veale EL, Striessnig J, Kelly E, … Pawson AJ (2021). The concise guide to pharmacology 2021/22: Ion channels. British Journal of Pharmacology, 178, S157–S245. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

