Injectable bioactive poly(propylene fumarate) and polycaprolactone based click chemistry bone cement for spinal fusion in rabbits
- PMID: 38644548
- PMCID: PMC11806930
- DOI: 10.1002/jbm.a.37725
Injectable bioactive poly(propylene fumarate) and polycaprolactone based click chemistry bone cement for spinal fusion in rabbits
Abstract
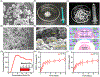
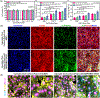
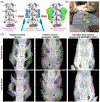
Degenerative spinal pathology is a widespread medical issue, and spine fusion surgeries are frequently performed. In this study, we fabricated an injectable bioactive click chemistry polymer cement for use in spinal fusion and bone regrowth. Taking advantages of the bioorthogonal click reaction, this cement can be crosslinked by itself eliminating the addition of a toxic initiator or catalyst, nor any external energy sources like UV light or heat. Furthermore, nano-hydroxyapatite (nHA) and microspheres carrying recombinant human bone morphogenetic protein-2 (rhBMP-2) and recombinant human vascular endothelial growth factor (rhVEGF) were used to make the cement bioactive for vascular induction and osteointegration. After implantation into a rabbit posterolateral spinal fusion (PLF) model, the cement showed excellent induction of new bone formation and bridging bone, achieving results comparable to autograft control. This is largely due to the osteogenic properties of nano-hydroxyapatite (nHA) and the released rhBMP-2 and rhVEGF growth factors. Since the availability of autograft sources is limited in clinical settings, this injectable bioactive click chemistry cement may be a promising alternative for spine fusion applications in addressing various spinal conditions.
Keywords: bioorthogonal click chemistry; bone cement; injectable polymers; spine fusion; tissue engineering.
© 2024 Wiley Periodicals LLC.
Conflict of interest statement
Dr. Elder is a consultant for DePuy Synthes and SI bone, owns stock options and is on the medical advisory board for Injectsense, and receives institutional support for clinical trials from Stryker and SI Bone. The other authors declare no competing financial interest.
Figures
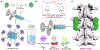


References
-
- Diebo BG, Shah NV, Boachie-Adjei O, et al. Adult spinal deformity. The Lancet. 2019;394(10193):160–172. - PubMed
-
- Martin BI, Mirza SK, Spina N, Spiker WR, Lawrence B, Brodke DS. Trends in lumbar fusion procedure rates and associated hospital costs for degenerative spinal diseases in the United States, 2004 to 2015. Spine. 2019;44(5):369–376. - PubMed
-
- Kamson S, Lu D, Sampson PD, Zhang Y. Full-endoscopic lumbar fusion outcomes in patients with minimal deformities: a retrospective study of data collected between 2011 and 2015. Pain Physician. 2019;22(1):75–88. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

