Relative Effectiveness of the MF59®-Adjuvanted Influenza Vaccine Versus High-Dose and Non-Adjuvanted Influenza Vaccines in Preventing Cardiorespiratory Hospitalizations During the 2019-2020 US Influenza Season
- PMID: 38644564
- PMCID: PMC11033326
- DOI: 10.1111/irv.13288
Relative Effectiveness of the MF59®-Adjuvanted Influenza Vaccine Versus High-Dose and Non-Adjuvanted Influenza Vaccines in Preventing Cardiorespiratory Hospitalizations During the 2019-2020 US Influenza Season
Abstract
Background: Adults ≥ 65 years of age have suboptimal influenza vaccination responses compared to younger adults due to age-related immunosenescence. Two vaccines were specifically developed to enhance protection: MF59-adjuvanted trivalent influenza vaccine (aIIV3) and high-dose egg-based trivalent influenza vaccine (HD-IIV3e).
Methods: In a retrospective cohort study conducted using US electronic medical records linked to claims data during the 2019-2020 influenza season, we compared the relative vaccine effectiveness (rVE) of aIIV3 with HD-IIV3e and a standard-dose non-adjuvanted egg-based quadrivalent inactivated influenza vaccine (IIV4e) for the prevention of cardiorespiratory hospitalizations, including influenza hospitalizations. We evaluated outcomes in the "any" diagnosis position and the "admitting" position on the claim. A doubly robust methodology using inverse probability of treatment weighting and logistic regression was used to adjust for covariate imbalance. rVE was calculated as 100 * (1 - ORadjusted).
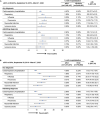
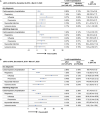
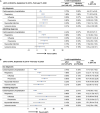
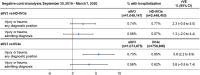
Results: The study included 4,299,594 adults ≥ 65 years of age who received aIIV3, HD-IIV3e, or IIV4e. Overall, aIIV3 was associated with lower proportions of cardiorespiratory hospitalizations with diagnoses in any position compared to HD-IIV3e (rVE = 3.9% [95% CI, 2.7-5.0]) or IIV4e (9.0% [95% CI, 7.7-10.4]). Specifically, aIIV3 was more effective compared with HD-IIV3e and IIV4e in preventing influenza hospitalizations (HD-IIV3e: 9.7% [95% CI, 1.9-17.0]; IIV4e: 25.3% [95% CI, 17.7-32.2]). Consistent trends were observed for admitting diagnoses.
Conclusion: Relative to both HD-IIV3e and IIV4e, aIIV3 provided improved protection from cardiorespiratory or influenza hospitalizations.
Keywords: adjuvanted influenza vaccine; high‐dose influenza vaccine; hospitalizations; influenza; ischemic stroke; myocardial infarction; older adults; pneumonia; quadrivalent inactivated influenza vaccine; relative vaccine effectiveness.
© 2024 The Authors. Influenza and Other Respiratory Viruses published by John Wiley & Sons Ltd.
Conflict of interest statement
M.I. and M.H. are employees of CSL Seqirus. J.P. has received honoraria as adviser, research activities, or conferences from Hipra, Novavax, Sanofi, and Seqirus. J.R.O. reports that his institution has received research grants from National Institutes of Health for influenza vaccine research. He has received honoraria from GSK, Moderna, Pfizer, and Seqirus to serve on scientific advisory boards. L.L.G. was an employee of Veradigm during the analysis execution and is currently an employee of BioCryst Pharmaceuticals, Inc. A.D. and M.B. are employees of Veradigm.
Figures
References
-
- National Center for Health Statistics , “Faststats: Influenza,” 2022, last accessed 29 April 2022, https://www.cdc.gov/nchs/fastats/flu.htm.
-
- Coleman B. L., Fadel S. A., Fitzpatrick T., and Thomas S. M., “Risk Factors for Serious Outcomes Associated With Influenza Illness in High‐ Versus Low‐ and Middle‐Income Countries: Systematic Literature Review and Meta‐Analysis,” Influenza and Other Respiratory Viruses 12, no. 1 (2018): 22–29, 10.1111/irv.12504. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

