Spontaneous and Masticatory Post-endodontic Pain After Using Endomethasone N vs SP Root Canal Sealers: A Randomised Controlled Clinical Trial
- PMID: 38644670
- PMCID: PMC11413606
- DOI: 10.14744/eej.2024.96977
Spontaneous and Masticatory Post-endodontic Pain After Using Endomethasone N vs SP Root Canal Sealers: A Randomised Controlled Clinical Trial
Abstract
Objective: Post-endodontic pain (PEP) after endodontic treatment (ET) might be reduced by adding cortisone to the composition of root canal sealer (RCS). This study aimed to test this hypothesis using grade A methodology.
Methods: A multicentric prospective randomised controlled clinical trial was performed in general practice. Adult patients with an indication of ET in a molar or premolar performed in one session were included be-tween 2021 and 2022 in 15 centres. The main objective was to demonstrate the superiority of Endomethasone N RCS (EndoN), compared to its hydrocortisone-free equivalent Endomethasone SP RCS (EndoSP), regarding the reduction of the maximum spontaneous PEP pain during the 7 days following the ET, self-estimated on a 0���100 mm Visual Analogic Scale (VAS). The secondary objectives were to assess 1) spontaneous PEP, 2) pro-voked (masticatory) PEP, 3) intake of analgesics, 4) quality of life and anxiety before and after ET, and 5) safety.
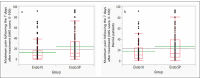
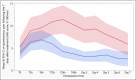
Results: The final sample consisted of 286 patients with a mean age of 47.7 years, including 51% men and 49% women. Before ET, 49.7% of the teeth were asymptomatic; provoked pain occurred in 29.4% and sponta-neous pain in 21.0%. The study evidenced a lower maximum spontaneous PEP intensity during the 7 days fol-lowing ET in EndoN compared to the EndoSP group (13.5+-17.9 vs 23.9+-26.6, IC 95% 10.5 [5.2���15.8], p=0.0001 Wilcoxon test). Maximal masticatory PEP was also lower in the EndoN group (12.3+-19.1 vs 24.0+-27.8, IC 95% 11.7 [5.8���17.6], p<0.0001 Wilcoxon test). At every evaluation time, the masticatory PEP in the EndoSP group was higher than in the EndoN group. In addition, no serious adverse events occurred during the study.
Conclusion: This RCT demonstrated EndoN's superiority over EndoSP in reducing spontaneous and mastica-tory PEP during the 7 days following ET. This study was funded by the Septodont company (Saint Maur des Foss��s, France) and registered at ClinicalTrials.gov # NCT04885686.
Conflict of interest statement
All authors declared no conflict of interest.
Figures

References
-
- Sathorn C, Parashos P, Messer H. The prevalence of postoperative pain and flare-up in single-and multiple-visit endodontic treatment: a systematic review. Int Endod J. 2008;41(2):91–9. - PubMed
-
- Arias A, de la Macorra JC, Hidalgo JJ, Azabal M. Predictive models of pain following root canal treatment: a prospective clinical study. Int Endod J. 2013;46(8):784–93. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

