Characterizing Prion-Like Protein Aggregation: Emerging Nanopore-Based Approaches
- PMID: 38644684
- PMCID: PMC11672191
- DOI: 10.1002/smtd.202400058
Characterizing Prion-Like Protein Aggregation: Emerging Nanopore-Based Approaches
Abstract
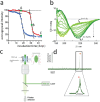
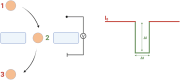
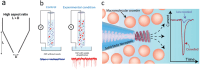
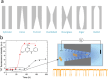
Prion-like protein aggregation is characteristic of numerous neurodegenerative diseases, such as Alzheimer's and Parkinson's diseases. This process involves the formation of aggregates ranging from small and potentially neurotoxic oligomers to highly structured self-propagating amyloid fibrils. Various approaches are used to study protein aggregation, but they do not always provide continuous information on the polymorphic, transient, and heterogeneous species formed. This review provides an updated state-of-the-art approach to the detection and characterization of a wide range of protein aggregates using nanopore technology. For each type of nanopore, biological, solid-state polymer, and nanopipette, discuss the main achievements for the detection of protein aggregates as well as the significant contributions to the understanding of protein aggregation and diagnostics.
Keywords: amyloid fibers; nanopore; prion‐like proteins oligomers; single‐molecule techniques.
© 2024 The Authors. Small Methods published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

