Diagnostic criteria and core outcome set development for necrotising otitis externa: the COSNOE Delphi consensus study
- PMID: 38644734
- PMCID: PMC11518678
- DOI: 10.1017/S0022215124000513
Diagnostic criteria and core outcome set development for necrotising otitis externa: the COSNOE Delphi consensus study
Abstract
Objective: Evidence for necrotising otitis externa (NOE) diagnosis and management is limited, and outcome reporting is heterogeneous. International best practice guidelines were used to develop consensus diagnostic criteria and a core outcome set (COS).
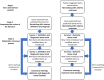
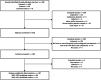
Methods: The study was pre-registered on the Core Outcome Measures in Effectiveness Trials (COMET) database. Systematic literature review identified candidate items. Patient-centred items were identified via a qualitative study. Items and their definitions were refined by multidisciplinary stakeholders in a two-round Delphi exercise and subsequent consensus meeting.
Results: The final COS incorporates 36 items within 12 themes: Signs and symptoms; Pain; Advanced Disease Indicators; Complications; Survival; Antibiotic regimes and side effects; Patient comorbidities; Non-antibiotic treatments; Patient compliance; Duration and cessation of treatment; Relapse and readmission; Multidisciplinary team management.Consensus diagnostic criteria include 12 items within 6 themes: Signs and symptoms (oedema, otorrhoea, granulation); Pain (otalgia, nocturnal otalgia); Investigations (microbiology [does not have to be positive], histology [malignancy excluded], positive CT and MRI); Persistent symptoms despite local and/or systemic treatment for at least two weeks; At least one risk factor for impaired immune response; Indicators of advanced disease (not obligatory but mut be reported when present at diagnosis). Stakeholders were unanimous that there is no role for secondary, graded, or optional diagnostic items. The consensus meeting identified themes for future research.
Conclusion: The adoption of consensus-defined diagnostic criteria and COS facilitates standardised research reporting and robust data synthesis. Inclusion of patient and professional perspectives ensures best practice stakeholder engagement.
Keywords: Otitis externa; diabetes mellitus; immunosuppression therapy; osteomyelitis.
Conflict of interest statement
None declared
Figures
References
-
- Chandler J. Malignant external otitis. Laryngoscope 1968;78:1257–94 - PubMed
-
- Bisbinas V, Stapleton E. Immunosuppression, infection, and epidermal compromise: an aetiological triad for necrotising otitis externa that highlights potential modifiable risk factors. J Laryngol Otol 2023;137:804–9 - PubMed
-
- Linton S, Stapleton E. Exploring theories for the exponential 16-year incidence rise of necrotising otitis externa in England. J Laryngol Otol 2022;136:925–9 - PubMed
-
- Byun Y, Patel J, Nguyen S, Lambert P. Necrotizing otitis externa: a systematic review and analysis of changing trends. Otol Neurotol 2020;41:1004–11 - PubMed
-
- Le Clerc N, Verillaud B, Duet M, Guichard J, Herman P, Kania R. Skull base osteomyelitis: incidence of resistance, morbidity, and treatment strategy. Laryngoscope 2014;124:2013–16 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources