Unveiling antioxidant capacity of standardized chitosan-tripolyphosphate microcapsules containing polyphenol-rich extract of Portulaca oleraceae
- PMID: 38644872
- PMCID: PMC11031833
- DOI: 10.1016/j.heliyon.2024.e29541
Unveiling antioxidant capacity of standardized chitosan-tripolyphosphate microcapsules containing polyphenol-rich extract of Portulaca oleraceae
Abstract
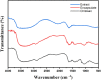
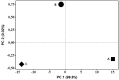
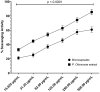
The medicinal plant Portulaca oleraceae has a long history of usage in traditional medicine. Plant extracts have several interesting pharmacological effects but have some drawbacks that can be addressed via capsulation with chitosan. This work set out to do just that tally up the antioxidant effects of a polyphenol-rich P. olerace extract and see how capsulation affected them. The reflux extraction and response surface methodology (RSM) were carried out to optimize the phenolic and flavonoid content of P. oleraceae extract. Additionally, high-resolution mass spectrometry was employed to determine the secondary metabolite present in the extract. The microcapsules of extract-loaded chitosan were prepared using the ionic gelation method and characterized in terms of size, encapsulation efficiency (EE), and morphology of microcapsules. Fourier transform infrared (FTIR) was used to observe the successful production of microcapsules with a principal component analysis (PCA) approach. The antioxidant activity of microcapsules was established using the radical scavenging method. According to RSM, the highest amounts of TPC and TFC were obtained at 72.894 % ethanol, 2.031 h, and 57.384 °C. The compounds were employed from the optimized extract of P. oleraceae including phenolics and flavonoids. The microcapsules were secured with a %EE of 43.56 ± 2.31 %. The characteristics of microcapsules were approved for the obtained product's successful synthesis according to the PCA. The microcapsules have antioxidant activity in a concentration-dependent manner (p < 0.0001). The findings of this study underscored the benefits of employing chitosan as a nanocarrier for extract, offering a promising approach to enhance plant-derived therapies.
Keywords: Antioxidant activity; Ionic gelation; Portulaca olearacea; Response surface methodology.
© 2024 The Authors. Published by Elsevier Ltd.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


References
-
- Twaij B.M., Hasan M.N. Bioactive secondary metabolites from plant sources: types, synthesis, and their therapeutic uses. Int. J. Plant Biol. 2022;13(1):4–14.
-
- Gandhi S.G., Mahajan V., Bedi Y.S. Changing trends in biotechnology of secondary metabolism in medicinal and aromatic plants. Planta. 2015;241(2):303–317. 2015. - PubMed
LinkOut - more resources
Full Text Sources

