Unraveling the structural complexity of and the effect of calcination temperature on calcium phosphates derived from Oreochromis niloticus bones
- PMID: 38644889
- PMCID: PMC11031838
- DOI: 10.1016/j.heliyon.2024.e29665
Unraveling the structural complexity of and the effect of calcination temperature on calcium phosphates derived from Oreochromis niloticus bones
Abstract
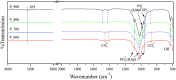
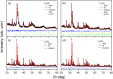
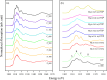
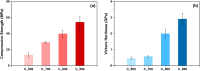
In this study, the interplay between the structural complexity, microstructure, and mechanical properties of calcium phosphates (CaPs) derived from fish bones, prepared at various calcination temperatures, and their corresponding sintered ceramics was explored. Fourier-transform infrared analysis revealed that the calcined powders primarily consisted of hydroxyapatite (HAp) and carbonated calcium hydroxyapatite, with an increasing concentration of Mg-substituted β-tricalcium phosphate (β-TCP) as the calcination temperature was increased. X-ray diffraction patterns showed enhanced sharpness of the peaks at higher temperatures, indicating a larger crystallite size and improved crystallinity. The ceramics exhibited a significantly larger crystallite size and an increased concentration of the β-TCP phase. Rietveld analysis revealed a larger volume of the β-TCP phase in the ceramics than in their calcined powders; this could be attributed to a newly formed β-TCP phase due to the decomposition of HAp. Extended X-ray absorption fine structure analysis revealed the incorporation of Mg in the Ca2 site of HAp, Ca2 site of β-TCP, and Ca5 site of β-TCP, with a higher substitution of Mg in the Ca5 site of β-TCP at elevated temperatures. The mechanical properties of HAp ceramics can be improved by increasing the calcination temperature because of their improved relative density and dense porous structure at elevated temperatures. This comprehensive investigation sheds light on the phase evolution, microstructural changes, and consequential impact on the mechanical properties of CaPs derived from fish bones, thereby facilitating the development of tailored CaP ceramics for biomedical applications.
Keywords: Hydroxyapatite; Mechanical properties; Microstructure; Phase composition; β-tricalcium phosphate.
© 2024 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



References
-
- Ressler A., Žužić A., Ivanišević I., Kamboj N., Ivanković H. Ionic substituted hydroxyapatite for bone regeneration applications: a review. Open Ceram. 2021;6 doi: 10.1016/j.oceram.2021.100122. - DOI
-
- Goto T., Sasaki K. Effects of trace elements in fish bones on crystal characteristics of hydroxyapatite obtained by calcination. Ceram. Int. 2014;40:10777–10785. doi: 10.1016/j.ceramint.2014.03.067. - DOI
-
- Wang P.E., Chaki T.K. Sintering behaviour and mechanical properties of hydroxyapatite and dicalcium phosphate. J. Mater. Sci. Mater. Med. 1993;4:150–158. doi: 10.1007/BF00120384. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

