Air-Stable, Large-Area 2D Metals and Semiconductors
- PMID: 38644964
- PMCID: PMC11027125
- DOI: 10.1021/acsnanoscienceau.3c00047
Air-Stable, Large-Area 2D Metals and Semiconductors
Abstract
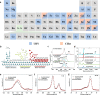
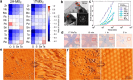
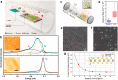
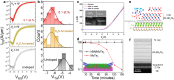
Two-dimensional (2D) materials are popular for fundamental physics study and technological applications in next-generation electronics, spintronics, and optoelectronic devices due to a wide range of intriguing physical and chemical properties. Recently, the family of 2D metals and 2D semiconductors has been expanding rapidly because they offer properties once unknown to us. One of the challenges to fully access their properties is poor stability in ambient conditions. In the first half of this Review, we briefly summarize common methods of preparing 2D metals and highlight some recent approaches for making air-stable 2D metals. Additionally, we introduce the physicochemical properties of some air-stable 2D metals recently explored. The second half discusses the air stability and oxidation mechanisms of 2D transition metal dichalcogenides and some elemental 2D semiconductors. Their air stability can be enhanced by optimizing growth temperature, substrates, and precursors during 2D material growth to improve material quality, which will be discussed. Other methods, including doping, postgrowth annealing, and encapsulation of insulators that can suppress defects and isolate the encapsulated samples from the ambient environment, will be reviewed.
© 2024 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

References
-
- Chaves A.; Azadani J. G.; Alsalman H.; da Costa D. R.; Frisenda R.; Chaves A. J.; Song S. H.; Kim Y. D.; He D.; Zhou J.; Castellanos-Gomez A.; Peeters F. M.; Liu Z.; Hinkle C. L.; Oh S. H.; Ye P. D.; Koester S. J.; Lee Y. H.; Avouris P.; Wang X.; Low T. Bandgap Engineering of Two-Dimensional Semiconductor Materials. NPJ. 2D Mater. Appl. 2020, 4 (1), 29.10.1038/s41699-020-00162-4. - DOI
-
- Lei Y.; Zhang T.; Lin Y.-C.; Granzier-Nakajima T.; Bepete G.; Kowalczyk D. A.; Lin Z.; Zhou D.; Schranghamer T. F.; Dodda A.; Sebastian A.; Chen Y.; Liu Y.; Pourtois G.; Kempa T. J.; Schuler B.; Edmonds M. T.; Quek S. Y.; Wurstbauer U.; Wu S. M.; Glavin N. R.; Das S.; Dash S. P.; Redwing J. M.; Robinson J. A.; Terrones M. Graphene and Beyond: Recent Advances in Two-Dimensional Materials Synthesis, Properties, and Devices. ACS Nanoscience Au 2022, 2 (6), 450–485. 10.1021/acsnanoscienceau.2c00017. - DOI - PMC - PubMed
-
- Manzeli S.; Ovchinnikov D.; Pasquier D.; Yazyev O. v.; Kis A. 2D Transition Metal Dichalcogenides. Nat. Rev. Mater. 2017, 2, 17033.10.1038/natrevmats.2017.33. - DOI
-
- Carvalho A.; Wang M.; Zhu X.; Rodin A. S.; Su H.; Castro Neto A. H. Phosphorene: From Theory to Applications. Nat. Rev. Mater. 2016, 1 (11), 1–16. 10.1038/natrevmats.2016.61. - DOI
-
- Nan H. Y.; Ni Z. H.; Wang J.; Zafar Z.; Shi Z. X.; Wang Y. Y. The Thermal Stability of Graphene in Air Investigated by Raman Spectroscopy. J. Raman Spectrosc. 2013, 44 (7), 1018–1021. 10.1002/jrs.4312. - DOI
Publication types
LinkOut - more resources
Full Text Sources
