Estradiol induces bone osteolysis in triple-negative breast cancer via its membrane-associated receptor ERα36
- PMID: 38644978
- PMCID: PMC11032217
- DOI: 10.1093/jbmrpl/ziae041
Estradiol induces bone osteolysis in triple-negative breast cancer via its membrane-associated receptor ERα36
Abstract
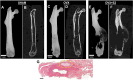
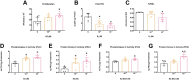
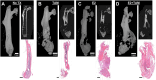
Triple-negative breast cancer (TNBC) is thought to be an estradiol-independent, hormone therapy-resistant cancer because of lack of estrogen receptor alpha 66 (ERα66). We identified a membrane-bound splice variant, ERα36, in TNBC cells that responds to estrogen (E2) and may contribute to bone osteolysis. We demonstrated that the MDA-MB-231 TNBC cell line, which expresses ERα36 similarly to MCF7 cells, is responsive to E2, forming osteolytic tumors in vivo. MDA-MB-231 cells activate osteoclasts in a paracrine manner. Conditioned media (CM) from MDA-MB-231 cells treated with bovine serum albumin-bound E2 (E2-BSA) increased activation of human osteoclast precursor cells; this was blocked by addition of anti-ERα36 antibody to the MDA-MB-231 cultures. Osteoclast activation and bone resorption genes were elevated in RAW 264.7 murine macrophages following treatment with E2-BSA-stimulated MDA-MB-231 CM. E2 and E2-BSA increased phospholipase C (PLC) and protein kinase C (PKC) activity in MDA-MB-231 cells. To examine the role of ERα36 signaling in bone osteolysis in TNBC, we used our bone-cancer interface mouse model in female athymic homozygous Foxn1nu mice. Mice with MDA-MB-231 tumors and treated with tamoxifen (TAM), E2, or TAM/E2 exhibited increased osteolysis, cortical bone breakdown, pathologic fracture, and tumor volume; the combined E2/TAM group also had reduced bone volume. These results suggest that E2 increased osteolytic lesions in TNBC through a membrane-mediated PLC/PKC pathway involving ERα36, which was enhanced by TAM, demonstrating the role of ERα36 and its membrane-associated signaling pathway in bone tumors. This work suggests that ERα36 may be a potential therapeutic target in patients with TNBC.
Keywords: ERα36; SERMs; bone resorption; bone–tumor interface; cross-talk; estrogen; osteoclasts; osteolysis; tumor–induced bone disease.
© The Author(s) 2024. Published by Oxford University Press on behalf of the American Society for Bone and Mineral Research.
Conflict of interest statement
None declared.
Figures





References
-
- Cetin K, Christiansen CF, Sværke C, Jacobsen JB, Sørensen HT. Survival in patients with breast cancer with bone metastasis: a Danish population-based cohort study on the prognostic impact of initial stage of disease at breast cancer diagnosis and length of the bone metastasis-free interval. BMJ Open. 2015;5(4):e007702. 10.1136/bmjopen-2015-007702 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

