Clinical Efficacy of Programmed Cell Death Ligand 1 Antibody in Treatment of Extranodal Natural Killer/T-Cell Lymphoma With Hemophagocytic Lymphohistiocytosis
- PMID: 38644986
- PMCID: PMC11027771
- DOI: 10.14740/jh1242
Clinical Efficacy of Programmed Cell Death Ligand 1 Antibody in Treatment of Extranodal Natural Killer/T-Cell Lymphoma With Hemophagocytic Lymphohistiocytosis
Abstract
Extranodal natural killer/T-cell lymphoma-associated hemophagocytic lymphohistiocytosis (ENKTCL-LAHS) is a rare disease with poor prognosis. Currently, there are no well-established treatments for LAHS. Almost 50% of patients experience relapsed or refractory disease to anti-hemophagocytic lymphohistiocytosis (HLH) treatment, and the regimen for salvage therapy is limited. We report a case of ENKTCL-LAHS that was successfully treated with a programmed cell death ligand 1 (PD-L1) antibody (sugemalimab) alone and provide a literature review on existing ENKTCL-LAHS treatment options. A 31-year-old man with relapsed ENKTCL complicated by HLH was admitted to our hospital. Following the administration of the PD-L1 antibody sugemalimab, fever was resolved, Epstein-Barr virus (EBV) DNA copy number was negative, and HLH-related blood biochemical markers were decreased in the patient. Consequently, the patient achieved complete remission with a progression-free time (PFS) of 44 months. The prognosis of ENKTCL-LAHS is extremely poor, and the clinical treatment of ENKTCL-HLH is challenging. No previous reports exist regarding the use of PD-L1 antibodies in ENKTCL-LAHS treatment. This study is the first to report a patient with ENKTCL-LAHS treated with the PD-L1 antibody alone, who achieved a long PFS of 44 months. Our results suggest the effectiveness and safety of sugemalimab in the treatment of ENKTCL-LAHS; however, more clinical cases are required for validation. The PD-L1 antibody presents a novel treatment option for patients with ENKTCL-LAHS and warrants further clinical promotion.
Keywords: HLH; LAHS; NK/T lymphoma; PD-L1 antibody; Treatment.
Copyright 2024, Yang et al.
Conflict of interest statement
The authors declare that this study was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
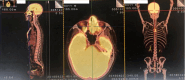
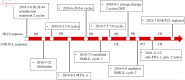
Figures

Similar articles
-
[Clinical characteristics and prognosis of lymphoma-associated hemophagocytic syndrome].Zhonghua Yi Xue Za Zhi. 2022 Jul 26;102(28):2173-2180. doi: 10.3760/cma.j.cn112137-20220221-00349. Zhonghua Yi Xue Za Zhi. 2022. PMID: 35872581 Chinese.
-
Multivariate analysis of prognosis for patients with natural killer/T cell lymphoma-associated hemophagocytic lymphohistiocytosis.Hematology. 2018 May;23(4):228-234. doi: 10.1080/10245332.2017.1385191. Epub 2017 Oct 6. Hematology. 2018. PMID: 28982299
-
Autologous Hematopoietic Stem Cell Transplantation for Patients with Lymphoma-Associated Hemophagocytic lymphohistiocytosis.Cell Transplant. 2021 Jan-Dec;30:9636897211057077. doi: 10.1177/09636897211057077. Cell Transplant. 2021. PMID: 34743574 Free PMC article.
-
Challenges in overcoming advanced-stage or relapsed refractory extranodal NK/T-cell lymphoma: meta-analysis of individual patient data.Front Oncol. 2024 Jul 31;14:1362367. doi: 10.3389/fonc.2024.1362367. eCollection 2024. Front Oncol. 2024. PMID: 39144825 Free PMC article.
-
Novel Immunotherapy Options for Extranodal NK/T-Cell Lymphoma.Front Oncol. 2018 Apr 30;8:139. doi: 10.3389/fonc.2018.00139. eCollection 2018. Front Oncol. 2018. PMID: 29761078 Free PMC article. Review.
References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
