Clinical Efficacy of Programmed Cell Death Ligand 1 Antibody in Treatment of Extranodal Natural Killer/T-Cell Lymphoma With Hemophagocytic Lymphohistiocytosis
- PMID: 38644986
- PMCID: PMC11027771
- DOI: 10.14740/jh1242
Clinical Efficacy of Programmed Cell Death Ligand 1 Antibody in Treatment of Extranodal Natural Killer/T-Cell Lymphoma With Hemophagocytic Lymphohistiocytosis
Abstract
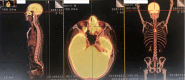
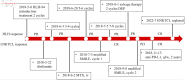
Extranodal natural killer/T-cell lymphoma-associated hemophagocytic lymphohistiocytosis (ENKTCL-LAHS) is a rare disease with poor prognosis. Currently, there are no well-established treatments for LAHS. Almost 50% of patients experience relapsed or refractory disease to anti-hemophagocytic lymphohistiocytosis (HLH) treatment, and the regimen for salvage therapy is limited. We report a case of ENKTCL-LAHS that was successfully treated with a programmed cell death ligand 1 (PD-L1) antibody (sugemalimab) alone and provide a literature review on existing ENKTCL-LAHS treatment options. A 31-year-old man with relapsed ENKTCL complicated by HLH was admitted to our hospital. Following the administration of the PD-L1 antibody sugemalimab, fever was resolved, Epstein-Barr virus (EBV) DNA copy number was negative, and HLH-related blood biochemical markers were decreased in the patient. Consequently, the patient achieved complete remission with a progression-free time (PFS) of 44 months. The prognosis of ENKTCL-LAHS is extremely poor, and the clinical treatment of ENKTCL-HLH is challenging. No previous reports exist regarding the use of PD-L1 antibodies in ENKTCL-LAHS treatment. This study is the first to report a patient with ENKTCL-LAHS treated with the PD-L1 antibody alone, who achieved a long PFS of 44 months. Our results suggest the effectiveness and safety of sugemalimab in the treatment of ENKTCL-LAHS; however, more clinical cases are required for validation. The PD-L1 antibody presents a novel treatment option for patients with ENKTCL-LAHS and warrants further clinical promotion.
Keywords: HLH; LAHS; NK/T lymphoma; PD-L1 antibody; Treatment.
Copyright 2024, Yang et al.
Conflict of interest statement
The authors declare that this study was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
