This is a preprint.
Disruption of Cholinergic Retinal Waves Alters Visual Cortex Development and Function
- PMID: 38644996
- PMCID: PMC11030223
- DOI: 10.1101/2024.04.05.588143
Disruption of Cholinergic Retinal Waves Alters Visual Cortex Development and Function
Abstract
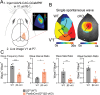
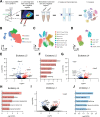
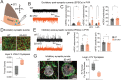
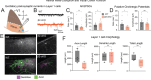
Retinal waves represent an early form of patterned spontaneous neural activity in the visual system. These waves originate in the retina before eye-opening and propagate throughout the visual system, influencing the assembly and maturation of subcortical visual brain regions. However, because it is technically challenging to ablate retina-derived cortical waves without inducing compensatory activity, the role these waves play in the development of the visual cortex remains unclear. To address this question, we used targeted conditional genetics to disrupt cholinergic retinal waves and their propagation to select regions of primary visual cortex, which largely prevented compensatory patterned activity. We find that loss of cholinergic retinal waves without compensation impaired the molecular and synaptic maturation of excitatory neurons located in the input layers of visual cortex, as well as layer 1 interneurons. These perinatal molecular and synaptic deficits also relate to functional changes observed at later ages. We find that the loss of perinatal cholinergic retinal waves causes abnormal visual cortex retinotopy, mirroring changes in the retinotopic organization of gene expression, and additionally impairs the processing of visual information. We further show that retinal waves are necessary for higher order processing of sensory information by impacting the state-dependent activity of layer 1 interneurons, a neuronal type that shapes neocortical state-modulation, as well as for state-dependent gain modulation of visual responses of excitatory neurons. Together, these results demonstrate that a brief targeted perinatal disruption of patterned spontaneous activity alters early cortical gene expression as well as synaptic and physiological development, and compromises both fundamental and, notably, higher-order functions of visual cortex after eye-opening.
Figures

Similar articles
-
Retinal Wave Patterns Are Governed by Mutual Excitation among Starburst Amacrine Cells and Drive the Refinement and Maintenance of Visual Circuits.J Neurosci. 2016 Mar 30;36(13):3871-86. doi: 10.1523/JNEUROSCI.3549-15.2016. J Neurosci. 2016. PMID: 27030771 Free PMC article.
-
Retinal waves coordinate patterned activity throughout the developing visual system.Nature. 2012 Oct 11;490(7419):219-25. doi: 10.1038/nature11529. Nature. 2012. PMID: 23060192 Free PMC article.
-
Wakefulness suppresses retinal wave-related neural activity in visual cortex.J Neurophysiol. 2017 Aug 1;118(2):1190-1197. doi: 10.1152/jn.00264.2017. Epub 2017 Jun 14. J Neurophysiol. 2017. PMID: 28615335 Free PMC article.
-
The role of early neural activity in the maturation of turtle retinal function.J Anat. 2001 Oct;199(Pt 4):375-83. doi: 10.1046/j.1469-7580.2001.19940375.x. J Anat. 2001. PMID: 11693298 Free PMC article. Review.
-
Spatiotemporal Features of Retinal Waves Instruct the Wiring of the Visual Circuitry.Front Neural Circuits. 2016 Jul 26;10:54. doi: 10.3389/fncir.2016.00054. eCollection 2016. Front Neural Circuits. 2016. PMID: 27507937 Free PMC article. Review.
References
-
- Antonini A, Stryker MP. 1996. Plasticity of geniculocortical afferents following brief or prolonged monocular occlusion in the cat. J Comp Neurol 369: 64–82 - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
