This is a preprint.
International Expert-Based Consensus Definition, Staging Criteria, and Minimum Data Elements for Osteoradionecrosis of the Jaw: An Inter-Disciplinary Modified Delphi Study
- PMID: 38645105
- PMCID: PMC11030490
- DOI: 10.1101/2024.04.07.24305400
International Expert-Based Consensus Definition, Staging Criteria, and Minimum Data Elements for Osteoradionecrosis of the Jaw: An Inter-Disciplinary Modified Delphi Study
Update in
-
International Expert-Based Consensus Definition, Classification Criteria, and Minimum Data Elements for Osteoradionecrosis of the Jaw: An Interdisciplinary Modified Delphi Study.Int J Radiat Oncol Biol Phys. 2025 Jun 1;122(2):341-354. doi: 10.1016/j.ijrobp.2024.12.017. Epub 2025 Jan 16. Int J Radiat Oncol Biol Phys. 2025. PMID: 39826846 Free PMC article.
Abstract
Purpose: Osteoradionecrosis of the jaw (ORNJ) is a severe iatrogenic disease characterized by bone death after radiation therapy (RT) to the head and neck. With over 9 published definitions and at least 16 diagnostic/staging systems, the true incidence and severity of ORNJ are obscured by lack of a standard for disease definition and severity assessment, leading to inaccurate estimation of incidence, reporting ambiguity, and likely under-diagnosis worldwide. This study aimed to achieve consensus on an explicit definition and phenotype of ORNJ and related precursor states through data standardization to facilitate effective diagnosis, monitoring, and multidisciplinary management of ORNJ.
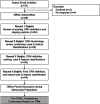
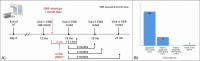
Methods: The ORAL Consortium comprised 69 international experts, including representatives from medical, surgical, radiation oncology, and oral/dental disciplines. Using a web-based modified Delphi technique, panelists classified descriptive cases using existing staging systems, reviewed systems for feature extraction and specification, and iteratively classified cases based on clinical/imaging feature combinations.
Results: The Consortium ORNJ definition was developed in alignment with SNOMED-CT terminology and recent ISOO-MASCC-ASCO guideline recommendations. Case review using existing ORNJ staging systems showed high rates of inability to classify (up to 76%). Ten consensus statements and nine minimum data elements (MDEs) were outlined for prospective collection and classification of precursor/ORNJ stages.
Conclusion: This study provides an international, consensus-based definition and MDE foundation for standardized ORNJ reporting in cancer survivors treated with RT. Head and neck surgeons, radiation, surgical, medical oncologists, and dental specialists should adopt MDEs to enable scalable health information exchange and analytics. Work is underway to develop both a human- and machine-readable knowledge representation for ORNJ (i.e., ontology) and multidisciplinary resources for dissemination to improve ORNJ reporting in academic and community practice settings.
Conflict of interest statement
The individual contributors/collaborators declare the following competing interests: National Institutes of Health (grants, travel, honoraria); Padagis (honoraria); NPi (honoraria); Castle Biosciences (consulting); Galera Therapeutics (consulting, grants); EMD Serono (advisory board, in-kind support, consulting, grants); UpToDate Inc (royalties); Cardinal Health (grants, consulting); Guy’s and St Thomas’ NHS Foundation Trust (grants); King’s College London (grants); Canadian Institutes of Health Research (grants); Canadian Foundation for Innovation (grants);Cancer Research Society (grants); Merck (advisory service, consulting, honoraria, travel, grants); Pfizer (stock, consulting, honoraria). Moderna (stock); Healthcare Services Group (stock); Dr. Reddy’s Laboratories (stock); CVS Health (stock). Organon (stock); Myomo (stock); Rewalk Robotics (stock); Elekta AB (grants, in-kind support, honoraria, travel); Philips Medical System (honoraria, travel); Varian/Siemens Healthineers (honoraria, travel). Kallsio, Inc. (royalties, licenses);Nanobiotix (consulting); LEO SAB (consulting, stock options);Shanghai JoAnn Medical Company(consulting); Yingming (consulting); Sanofi-Regeneron (honoraria); Merck Sharp & Dohme (honoraria); Glaxo Smith Kline (honoraria); Merus (honoraria); Sun Pharma (honoraria); Angelini (honoraria, consulting); MeiraGtx (grants);PCCA (grants); Mureva (grants); K pharmaceuticals (honoraria, consulting); Lipella Pharmaceuticals (honoraria, consulting); Amgen (honoraria, consulting); Bristol Myers Squibb (grants); Debiopharm (grants);ACI Clinical (consulting);Genentech (consulting); Astellas (consulting); Immunitas (consulting, stock); SIRPant (consulting); LEK (consulting); Burns and White (expert testimony); Doximity (stock).
Figures


References
-
- Watson E. E. et al. Development and Standardization of a Classification System for Osteoradionecrosis: Implementation of a Risk-Based Model. medRxiv 2023.09.12.23295454 (2023) doi: 10.1101/2023.09.12.23295454. - DOI
-
- Dijk L. V. van et al. Normal Tissue Complication Probability (NTCP) prediction model for osteoradionecrosis of the mandible in head and neck cancer patients following radiotherapy: Large-scale observational cohort. medRxiv 2021.03.04.21252505 (2021) doi: 10.1101/2021.03.04.21252505. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
