This is a preprint.
Generalizability of Tau and Amyloid Plasma Biomarkers in Alzheimer's Disease Cohorts of Diverse Genetic Ancestries
- PMID: 38645114
- PMCID: PMC11030471
- DOI: 10.1101/2024.04.10.24305617
Generalizability of Tau and Amyloid Plasma Biomarkers in Alzheimer's Disease Cohorts of Diverse Genetic Ancestries
Update in
-
Generalizability of tau and amyloid plasma biomarkers in Alzheimer's disease cohorts of diverse genetic ancestries.Alzheimers Dement. 2025 Mar;21(3):e14367. doi: 10.1002/alz.14367. Alzheimers Dement. 2025. PMID: 40133765 Free PMC article.
Abstract
Introduction: Plasma phosphorylated threonine-181 of Tau and amyloid beta are biomarkers for differential diagnosis and preclinical detection of Alzheimer disease (AD). Given differences in AD risk across diverse populations, generalizability of existing biomarker data is not assured.
Methods: In 2,086 individuals of diverse genetic ancestries (African American, Caribbean Hispanic, and Peruvians) we measured plasma pTau-181 and Aβ42/Aβ40. Differences in biomarkers between cohorts and clinical diagnosis groups and the potential discriminative performance of the two biomarkers were assessed.
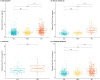
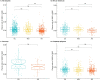
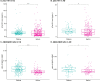
Results: pTau-181 and Aβ42/Aβ40 were consistent across cohorts. Higher levels of pTau181 were associated with AD while Aβ42/Aβ40 had minimal differences. Correspondingly, pTau-181 had greater predictive value than Aβ42/Aβ40, however, the area under the curve differed between cohorts.
Discussion: pTau-181 as a plasma biomarker for clinical AD is generalizable across genetic ancestries, but predictive value may differ. Combining genomic and biomarker data from diverse individuals will increase understanding of genetic risk and refine clinical diagnoses.
Keywords: Alzheimer’s disease; amyloid; diverse ancestry; plasma biomarkers; tau.
Conflict of interest statement
Conflict of Interest The authors declare no conflicts of interest.
Figures

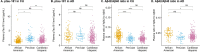

Similar articles
-
Generalizability of tau and amyloid plasma biomarkers in Alzheimer's disease cohorts of diverse genetic ancestries.Alzheimers Dement. 2025 Mar;21(3):e14367. doi: 10.1002/alz.14367. Alzheimers Dement. 2025. PMID: 40133765 Free PMC article.
-
The Bio-Hermes Study: Biomarker database developed to investigate blood-based and digital biomarkers in community-based, diverse populations clinically screened for Alzheimer's disease.Alzheimers Dement. 2024 Apr;20(4):2752-2765. doi: 10.1002/alz.13722. Epub 2024 Feb 28. Alzheimers Dement. 2024. PMID: 38415908 Free PMC article.
-
Exploring the ability of plasma pTau217, pTau181 and beta-amyloid in mirroring cerebrospinal fluid biomarker profile of Mild Cognitive Impairment by the fully automated Lumipulse® platform.Fluids Barriers CNS. 2025 Jan 21;22(1):9. doi: 10.1186/s12987-025-00620-5. Fluids Barriers CNS. 2025. PMID: 39838411 Free PMC article.
-
Blood-based biomarkers for Alzheimer's disease in Down syndrome: A systematic review and meta-analysis.Alzheimers Dement. 2025 Apr;21(4):e70135. doi: 10.1002/alz.70135. Alzheimers Dement. 2025. PMID: 40219863 Free PMC article.
-
CSF and plasma biomarkers in cerebral amyloid angiopathy: A single-center study and a systematic review/meta-analysis.Eur Stroke J. 2025 Mar;10(1):278-288. doi: 10.1177/23969873241260538. Epub 2024 Jun 13. Eur Stroke J. 2025. PMID: 38869035 Free PMC article.
References
-
- 2023 Alzheimer’s disease facts and figures. Alzheimer’s & Dementia 2023;19:1598–1695. - PubMed
-
- Beecham G.W., Vardarajan B., Blue E., Bush W., Jaworski J., Barral S., DeStefano A., Hamilton-Nelson K., Kunkle B., Martin E.R., Naj A., Rajabli F., Reitz C., Thornton T., van Duijn C., Goate A., Seshadri S., Farrer L.A., Boerwinkle E., Schellenberg G., Haines J.L., Wijsman E., Mayeux R., Pericak-Vance M.A., Alzheimer’s Disease Sequencing Project, Rare genetic variation implicated in non-Hispanic white families with Alzheimer disease. Neurol Genet 2018;4:e286. - PMC - PubMed
-
- Bis J.C., Jian X., Kunkle B.W., Chen Y., Hamilton-Nelson K.L., Bush W.S., Salerno W.J., Lancour D., Ma Y., Renton A.E., Marcora E., Farrell J.J., Zhao Y., Qu L., Ahmad S., Amin N., Amouyel P., Beecham G.W., Below J.E., Campion D., Charbonnier C., Chung J., Crane P.K., Cruchaga C., Cupples L.A., Dartigues J.F., Debette S., Deleuze J.F., Fulton L., Gabriel S.B., Genin E., Gibbs R.A., Goate A., Grenier-Boley B., Gupta N., Haines J.L., Havulinna A.S., Helisalmi S., Hiltunen M., Howrigan D.P., Ikram M.A., Kaprio J., Konrad J., Kuzma A., Lander E.S., Lathrop M., Lehtimaki T., Lin H., Mattila K., Mayeux R., Muzny D.M., Nasser W., Neale B., Nho K., Nicolas G., Patel D., Pericak-Vance M.A., Perola M., Psaty B.M., Quenez O., Rajabli F., Redon R., Reitz C., Remes A.M., Salomaa V., Sarnowski C., Schmidt H., Schmidt M., Schmidt R., Soininen H., Thornton T.A., Tosto G., Tzourio C., van der Lee S.J., van Duijn C.M., Vardarajan B., Wang W., Wijsman E., Wilson R.K., Witten D., Worley K.C., Zhang X., Alzheimer’s Disease Sequencing Project, Bellenguez C., Lambert J.C., Kurki M.I., Palotie A., Daly M., Boerwinkle E., Lunetta K.L., Destefano A.L., Dupuis J., Martin E.R., Schellenberg G.D., Seshadri S., Naj A.C., Fornage M., Farrer L.A., Whole exome sequencing study identifies novel rare and common Alzheimer’s-Associated variants involved in immune response and transcriptional regulation. Mol Psychiatry 2018. - PMC - PubMed
-
- Vardarajan B.N., Barral S., Jaworski J., Beecham G.W., Blue E., Tosto G., Reyes-Dumeyer D., Medrano M., Lantigua R., Naj A., Thornton T., DeStefano A., Martin E., Wang L.S., Brown L., Bush W., van Duijn C., Goate A., Farrer L., Haines J.L., Boerwinkle E., Schellenberg G., Wijsman E., Pericak-Vance M.A., Mayeux R., Alzheimer’s Disease Sequencing Project, Wang L.S., Whole genome sequencing of Caribbean Hispanic families with late-onset Alzheimer’s disease. Ann Clin Transl Neurol 2018;5:406–417. - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
