This is a preprint.
Impact of Obesity on the CCR6-CCL20 Axis in Epidermal γδ T Cells and IL-17A Production in Murine Wound Healing and Psoriasis
- PMID: 38645150
- PMCID: PMC11030331
- DOI: 10.1101/2024.04.09.588780
Impact of Obesity on the CCR6-CCL20 Axis in Epidermal γδ T Cells and IL-17A Production in Murine Wound Healing and Psoriasis
Update in
-
Impact of obesity on the CCR6-CCL20 axis in epidermal γδ T cells and IL-17A production in murine wound healing and psoriasis.J Immunol. 2025 Jan 1;214(1):153-166. doi: 10.1093/jimmun/vkae011. J Immunol. 2025. PMID: 40073267 Free PMC article.
Abstract
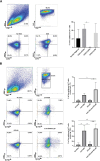
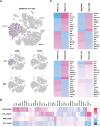
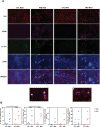
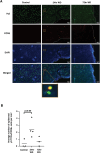
Obesity is associated with comorbidities including type 2 diabetes, chronic nonhealing wounds and psoriasis. Normally skin homeostasis and repair is regulated through the production of cytokines and growth factors derived from skin-resident cells including epidermal γδ T cells. However epidermal γδ T cells exhibit reduced proliferation and defective growth factor and cytokine production during obesity and type 2 diabetes. One of the genes modulated in epidermal γδ T cells during obesity and type 2 diabetes is CCR6, which is the receptor for CCL20. CCL20 is elevated in the skin during obesity and type 2 diabetes. Here we identify a subset of murine epidermal γδ T cells that expresses CCR6 in response to activation in vitro and post-wounding or psoriasis induction with imiquimod in vivo. We show that CCL20 stimulates epidermal γδ T cells to produce IL-17 suggesting CCR6 regulates the IL-17 axis as in dermal γδ T cells. Further, epidermal γδ T cells upregulate CCR6 and produce IL-17 during murine models of wound repair and psoriasis. Obesity increases CCR6 and IL-17 expression by epidermal γδ T cells during wound repair but has less of an effect during psoriasis. These findings have novel implications for the regulation of a specific population of IL-17-producing epidermal γδ T cells during skin damage and inflammation.
Figures

Similar articles
-
Impact of obesity on the CCR6-CCL20 axis in epidermal γδ T cells and IL-17A production in murine wound healing and psoriasis.J Immunol. 2025 Jan 1;214(1):153-166. doi: 10.1093/jimmun/vkae011. J Immunol. 2025. PMID: 40073267 Free PMC article.
-
Hyperforin Ameliorates Imiquimod-Induced Psoriasis-Like Murine Skin Inflammation by Modulating IL-17A-Producing γδ T Cells.Front Immunol. 2021 May 5;12:635076. doi: 10.3389/fimmu.2021.635076. eCollection 2021. Front Immunol. 2021. PMID: 34025642 Free PMC article.
-
GRP78 Downregulation in Keratinocytes Promotes Skin Inflammation through the Recruitment and Activation of CCR6+ IL-17A-Producing γδ T Cells.J Invest Dermatol. 2024 Jul;144(7):1557-1567.e11. doi: 10.1016/j.jid.2023.12.023. Epub 2024 Jan 24. J Invest Dermatol. 2024. PMID: 38272207
-
The CCL20 and CCR6 axis in psoriasis.Scand J Immunol. 2020 Mar;91(3):e12846. doi: 10.1111/sji.12846. Epub 2019 Nov 24. Scand J Immunol. 2020. PMID: 31692008 Review.
-
Functions of Vγ4 T Cells and Dendritic Epidermal T Cells on Skin Wound Healing.Front Immunol. 2018 Jun 4;9:1099. doi: 10.3389/fimmu.2018.01099. eCollection 2018. Front Immunol. 2018. PMID: 29915573 Free PMC article. Review.
References
-
- Loots M. A., Lamme E. N., Mekkes J. R., Bos J. D., and Middelkoop E.. 1999. Cultured fibroblasts from chronic diabetic wounds on the lower extremity (non-insulin-dependent diabetes mellitus) show disturbed proliferation. Arch. Dermatol. Res. 291: 93–99 - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
