This is a preprint.
Factors associated with tuberculosis treatment initiation among bacteriologically negative individuals evaluated for tuberculosis: an individual patient data meta-analysis
- PMID: 38645191
- PMCID: PMC11030305
- DOI: 10.1101/2024.04.07.24305445
Factors associated with tuberculosis treatment initiation among bacteriologically negative individuals evaluated for tuberculosis: an individual patient data meta-analysis
Update in
-
Factors associated with tuberculosis treatment initiation among bacteriologically negative individuals evaluated for tuberculosis: An individual patient data meta-analysis.PLoS Med. 2025 Jan 13;22(1):e1004502. doi: 10.1371/journal.pmed.1004502. eCollection 2025 Jan. PLoS Med. 2025. PMID: 39804959 Free PMC article.
Abstract
Background: Globally, over one-third of pulmonary tuberculosis (TB) disease diagnoses are made based on clinical criteria after a negative diagnostic test result. Understanding factors associated with clinicians' decisions to initiate treatment for individuals with negative test results is critical for predicting the potential impact of new diagnostics.
Methods: We performed a systematic review and individual patient data meta-analysis using studies conducted between January/2010 and December/2022 (PROSPERO: CRD42022287613). We included trials or cohort studies that enrolled individuals evaluated for TB in routine settings. In these studies participants were evaluated based on clinical examination and routinely-used diagnostics, and were followed for ≥1 week after the initial test result. We used hierarchical Bayesian logistic regression to identify factors associated with treatment initiation following a negative result on an initial bacteriological test (e.g., sputum smear microscopy, Xpert MTB/RIF).
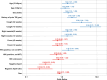
Findings: Multiple factors were positively associated with treatment initiation: male sex [adjusted Odds Ratio (aOR) 1.61 (1.31-1.95)], history of prior TB [aOR 1.36 (1.06-1.73)], reported cough [aOR 4.62 (3.42-6.27)], reported night sweats [aOR 1.50 (1.21-1.90)], and having HIV infection but not on ART [aOR 1.68 (1.23-2.32)]. Treatment initiation was substantially less likely for individuals testing negative with Xpert [aOR 0.77 (0.62-0.96)] compared to smear microscopy and declined in more recent years.
Interpretation: Multiple factors influenced decisions to initiate TB treatment despite negative test results. Clinicians were substantially less likely to treat in the absence of a positive test result when using more sensitive, PCR-based diagnostics.
Figures

References
-
- World Health Organization. Global Tuberculosis Report 2023 [Internet]. 2023. [cited 2024 Jan 15]. Available from: https://www.who.int/teams/global-tuberculosis-programme/tb-reports/globa...
-
- World Health Organization. The end TB strategy [Internet]. 2015. Available from: https://www.who.int/publications-detail-redirect/WHO-HTM-TB-2015.19
-
- World Health Organization. WHO consolidated guidelines on tuberculosis. Module 3: Diagnosis - Rapid diagnostics for tuberculosis detection 2021 update [Internet]. 2021. Available from: https://www.who.int/publications-detail-redirect/9789240029415 - PubMed
-
- Hong JM, Lee H, Menon NV, Lim CT, Lee LP, Ong CWM. Point-of-care diagnostic tests for tuberculosis disease. Science Translational Medicine. 2022. Apr 6;14(639):eabj4124. - PubMed
-
- Calligaro GL, Theron G, Khalfey H, Peter J, Meldau R, Matinyenya B, et al. Burden of tuberculosis in intensive care units in Cape Town, South Africa, and assessment of the accuracy and effect on patient outcomes of the Xpert MTB/RIF test on tracheal aspirate samples for diagnosis of pulmonary tuberculosis: a prospective burden of disease study with a nested randomised controlled trial. Lancet Respir Med. 2015. Aug;3(8):621–30. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
