Effects of N-glycans on the structure of human IgA2
- PMID: 38645274
- PMCID: PMC11026580
- DOI: 10.3389/fmolb.2024.1390659
Effects of N-glycans on the structure of human IgA2
Abstract
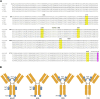
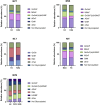
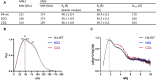
The transition of IgA antibodies into clinical development is crucial because they have the potential to create a new class of therapeutics with superior pathogen neutralization, cancer cell killing, and immunomodulation capacity compared to IgG. However, the biological role of IgA glycans in these processes needs to be better understood. This study provides a detailed biochemical, biophysical, and structural characterization of recombinant monomeric human IgA2, which varies in the amount/locations of attached glycans. Monomeric IgA2 antibodies were produced by removing the N-linked glycans in the CH1 and CH2 domains. The impact of glycans on oligomer formation, thermal stability, and receptor binding was evaluated. In addition, we performed a structural analysis of recombinant IgA2 in solution using Small Angle X-Ray Scattering (SAXS) to examine the effect of glycans on protein structure and flexibility. Our results indicate that the absence of glycans in the Fc tail region leads to higher-order aggregates. SAXS, combined with atomistic modeling, showed that the lack of glycans in the CH2 domain results in increased flexibility between the Fab and Fc domains and a different distribution of open and closed conformations in solution. When binding with the Fcα-receptor, the dissociation constant remains unaltered in the absence of glycans in the CH1 or CH2 domain, compared to the fully glycosylated protein. These results provide insights into N-glycans' function on IgA2, which could have important implications for developing more effective IgA-based therapeutics in the future.
Keywords: IgA antibodies; N-linked glycan; SAXS; flexibility; protein assembly; protein stability.
Copyright © 2024 Ruocco, Grünwald-Gruber, Rad, Tscheliessnig, Hammel and Strasser.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures




References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

