Study of Direct N7 Regioselective tert-Alkylation of 6-Substituted Purines and Their Modification at Position C6 through O, S, N, and C Substituents
- PMID: 38645315
- PMCID: PMC11024948
- DOI: 10.1021/acsomega.4c00068
Study of Direct N7 Regioselective tert-Alkylation of 6-Substituted Purines and Their Modification at Position C6 through O, S, N, and C Substituents
Abstract
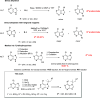
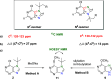
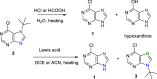
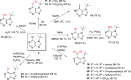
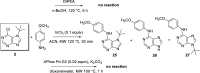
A new N7 direct regioselective method allowing the introduction of tert-alkyl groups into appropriate 6-substituted purine derivatives is developed. This method is based on a reaction of N-trimethylsilylated purines with a tert-alkyl halide using SnCl4 as a catalyst. In this work, we study the structure and optimal reaction conditions leading to the N7 isomer and in some cases also to the N9 isomer. The main goal is devoted to preparing 7-(tert-butyl)-6-chloropurine as a suitable compound for other purine transformations. The stability of the tert-butyl group at the N7 position is tested for classic model reactions, leading to the preparation of new 6,7-disubstituted purine derivatives, which is also interesting from the point of view of possible biological activity.
© 2024 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

Similar articles
-
Study of the N7 Regioselective Glycosylation of 6-Chloropurine and 2,6-Dichloropurine with Tin and Titanium Tetrachloride.J Org Chem. 2021 Oct 1;86(19):13265-13275. doi: 10.1021/acs.joc.1c01186. Epub 2021 Sep 16. J Org Chem. 2021. PMID: 34528791
-
Microwave promoted C6-alkylation of purines through S(N)Ar-based reaction of 6-chloropurines with 3-alkyl-acetylacetone.Org Biomol Chem. 2011 Apr 7;9(7):2065-8. doi: 10.1039/c0ob01213k. Epub 2011 Feb 21. Org Biomol Chem. 2011. PMID: 21340084
-
N9-Substituted N⁶-[(3-methylbut-2-en-1-yl)amino]purine derivatives and their biological activity in selected cytokinin bioassays.Bioorg Med Chem. 2011 Dec 1;19(23):7244-51. doi: 10.1016/j.bmc.2011.09.052. Epub 2011 Oct 5. Bioorg Med Chem. 2011. PMID: 22019467
-
Transformation of mutagenic aromatic amines into non-mutagenic species by alkyl substituents. Part II: alkylation far away from the amino function.Mutat Res. 2002 Mar 25;515(1-2):15-38. doi: 10.1016/s1383-5718(01)00345-x. Mutat Res. 2002. PMID: 11909752
-
2,3-Dicyclohexylsuccinimide as a directing/protecting group for the regioselective glycosylation or alkylation of purines.Chem Commun (Camb). 2013 Apr 11;49(28):2936-8. doi: 10.1039/c3cc37265k. Chem Commun (Camb). 2013. PMID: 23459620
Cited by
-
Sulfur-Mediated Ring-Opening Cyclization of Spirocyclopropanes for the Construction of Benzo[b]thiophene Skeleton.Chem Asian J. 2025 Aug;20(16):e00262. doi: 10.1002/asia.202500262. Epub 2025 May 14. Chem Asian J. 2025. PMID: 40366180 Free PMC article.
-
Synthesis of Purine-1,4,7,10-Tetraazacyclododecane Conjugate and Its Complexation Modes with Copper(II).Molecules. 2025 Apr 4;30(7):1612. doi: 10.3390/molecules30071612. Molecules. 2025. PMID: 40286228 Free PMC article.
References
-
- Duke C. C.; Liepa A. J.; MacLeod J. K.; Letham D. S.; Parker C. W. Synthesis of Raphanatin and Its 6-Benzylaminopurine Analogue. J. Chem. Soc. Chem. Commun. 1975, (24), 964.10.1039/c39750000964. - DOI
-
- Xavier N. M.; Goncalves-Pereira R.; Jorda R.; Hendrychová D.; Oliveira M. C. Novel Dodecyl-Containing Azido and Glucuronamide-Based Nucleosides Exhibiting Anticancer Potential. Pure Appl. Chem. 2019, 91 (7), 1085–1105. 10.1515/pac-2019-0106. - DOI
LinkOut - more resources
Full Text Sources
