Synthesis and Evaluation of [64Cu]Cu-NOTA-HFn for PET Imaging of Transferrin Receptor 1 Expression in Nasopharyngeal Carcinoma
- PMID: 38645324
- PMCID: PMC11024937
- DOI: 10.1021/acsomega.4c00187
Synthesis and Evaluation of [64Cu]Cu-NOTA-HFn for PET Imaging of Transferrin Receptor 1 Expression in Nasopharyngeal Carcinoma
Abstract
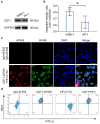
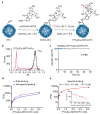
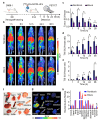
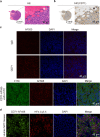
As recurrent and metastatic nasopharyngeal carcinoma (NPC) is the most common cause of death among patients with NPC, there is an urgent clinical need for the development of precision diagnosis to guide personalized treatment. Recent emerging evidence substantiates the increased expression of transferrin receptor 1 (also known as cluster of differentiation 71, CD71) within tumor tissues and the inherent targeting capability of natural heavy-chain ferritin (HFn) toward CD71. This study aimed to synthesize and assess a radiotracer ([64Cu]Cu-NOTA-HFn) designed to target CD71 for positron emission tomography (PET) imaging in an NPC tumor-bearing mouse model. The entire radiolabeling process of [64Cu]Cu-NOTA-HFn was completed within 15 min with high yield (>98.5%) and high molar activity (72.96 ± 21.33 GBq/μmol). The in vitro solubility and stability experiments indicated that [64Cu]Cu-NOTA-HFn had a high water solubility (log P = -2.42 ± 0.52, n = 6) and good stability in phosphate-buffered saline (PBS) for up to 48 h. The cell saturation binding assay indicated that [64Cu]Cu-NOTA-HFn had a nanomolar affinity (Kd = 10.9 ± 6.1 nM) for CD71-overexpressing C666-1 cells. To test the target engagement in vivo, prolonged-time PET imaging was performed at 1, 6, 12, 24, and 36 h postinjection (p.i.) of [64Cu]Cu-NOTA-HFn to C666-1 NPC tumor-bearing mice. The C666-1 tumors could be visualized by [64Cu]Cu-NOTA-HFn and blocked by nonradiolabeled HFn. PET imaging quantitative analysis demonstrated that the uptake of [64Cu]Cu-NOTA-HFn in C666-1 tumors peaked at 6 h p.i. and the best radioactive tumor-to-muscle ratio was 10.53 ± 3.11 (n = 3). Ex vivo biodistribution assay at 6 h p.i. showed that the tumor uptakes were 1.43 ± 0.23%ID/g in the nonblock group and 0.92 ± 0.2%ID/g in the block group (n = 3, p < 0.05). Immunohistochemistry and immunofluorescence staining confirmed positive expression of CD71 and the uptake of HFn in C666-1 tumor tissues. In conclusion, our experiments demonstrated that [64Cu]Cu-NOTA-HFn possesses a very high target engagement for CD71-positive NPC tumors and provided a fundamental basis for further clinical translation.
© 2024 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
Similar articles
-
Preclinical Evaluation of [64Cu]NOTA-CP01 as a PET Imaging Agent for Metastatic Esophageal Squamous Cell Carcinoma.Mol Pharm. 2021 Sep 6;18(9):3638-3648. doi: 10.1021/acs.molpharmaceut.1c00600. Epub 2021 Aug 23. Mol Pharm. 2021. PMID: 34424706
-
Automated Synthesis and Preclinical Evaluation of Optimized Integrin α6-Targeted Positron Emission Tomography Imaging of Pancreatic Cancer.Mol Pharm. 2023 Aug 7;20(8):4277-4284. doi: 10.1021/acs.molpharmaceut.3c00321. Epub 2023 Jul 18. Mol Pharm. 2023. PMID: 37463487
-
ImmunoPET Imaging of CD146 in Murine Models of Intrapulmonary Metastasis of Non-Small Cell Lung Cancer.Mol Pharm. 2017 Oct 2;14(10):3239-3247. doi: 10.1021/acs.molpharmaceut.7b00216. Epub 2017 Sep 6. Mol Pharm. 2017. PMID: 28825843 Free PMC article.
-
PET imaging of insulin-like growth factor type 1 receptor expression with a 64Cu-labeled Affibody molecule.Amino Acids. 2015 Jul;47(7):1409-19. doi: 10.1007/s00726-015-1975-4. Epub 2015 Apr 9. Amino Acids. 2015. PMID: 25854877
-
Cargo loading within ferritin nanocages in preparation for tumor-targeted delivery.Nat Protoc. 2021 Oct;16(10):4878-4896. doi: 10.1038/s41596-021-00602-5. Epub 2021 Sep 8. Nat Protoc. 2021. PMID: 34497386 Review.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
