A miniaturized multicellular platform to mimic the 3D structure of the alveolar-capillary barrier
- PMID: 38646009
- PMCID: PMC11026571
- DOI: 10.3389/fbioe.2024.1346660
A miniaturized multicellular platform to mimic the 3D structure of the alveolar-capillary barrier
Abstract
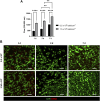
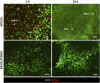
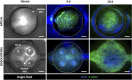
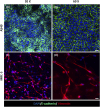
Several diseases affect the alveoli, and the efficacy of medical treatments and pharmaceutical therapies is hampered by the lack of pre-clinical models able to recreate in vitro the diseases. Microfluidic devices, mimicking the key structural and compositional features of the alveoli, offer several advantages to medium and high-throughput analysis of new candidate therapies. Here, we developed an alveolus-on-a-chip recapitulating the microanatomy of the physiological tissue by including the epithelium, the fibrous interstitial layer and the capillary endothelium. A PDMS device was obtained assembling a top layer and a bottom layer obtained by replica molding. A polycaprolactone/gelatin (PCL-Gel) electrospun membrane was included within the two layers supporting the seeding of 3 cell phenotypes. Epithelial cells were grown on a fibroblast-laden collagen hydrogel located on the top side of the PCL-Gel mats while endothelial cells were seeded on the basolateral side of the membrane. The innovative design of the microfluidic device allows to replicate both cell-cell and cell-extracellular matrix interactions according to the in vivo cell arrangement along with the establishment of physiologically relevant air-liquid interface conditions. Indeed, high cell viability was confirmed for up to 10 days and the formation of a tight endothelial and epithelial barrier was assessed by immunofluorescence assays.
Keywords: ECM-like substrate; alveolar-capillary barrier; alveolus-on-a-chip; cell tri-culture; in vitro models.
Copyright © 2024 Licciardello, Traldi, Cicolini, Bertana, Marasso, Cocuzza, Tonda-Turo and Ciardelli.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures



Similar articles
-
Development of biomimetic co-culture and tri-culture models to mimic the complex structure of the alveolar-capillary barrier.Biomater Adv. 2023 Nov;154:213620. doi: 10.1016/j.bioadv.2023.213620. Epub 2023 Sep 8. Biomater Adv. 2023. PMID: 37690344
-
Triple co-culture of human alveolar epithelium, endothelium and macrophages for studying the interaction of nanocarriers with the air-blood barrier.Acta Biomater. 2019 Jun;91:235-247. doi: 10.1016/j.actbio.2019.04.037. Epub 2019 Apr 18. Acta Biomater. 2019. PMID: 31004840
-
Microfluidic lung airway-on-a-chip with arrayable suspended gels for studying epithelial and smooth muscle cell interactions.Lab Chip. 2018 May 1;18(9):1298-1309. doi: 10.1039/c7lc01357d. Lab Chip. 2018. PMID: 29651473
-
A novel organ-chip system emulates three-dimensional architecture of the human epithelia and the mechanical forces acting on it.Biomaterials. 2021 Aug;275:120957. doi: 10.1016/j.biomaterials.2021.120957. Epub 2021 Jun 6. Biomaterials. 2021. PMID: 34130145
-
Engineering Tissue Barrier Models on Hydrogel Microfluidic Platforms.ACS Appl Mater Interfaces. 2021 Mar 31;13(12):13920-13933. doi: 10.1021/acsami.0c21573. Epub 2021 Mar 19. ACS Appl Mater Interfaces. 2021. PMID: 33739812 Review.
References
LinkOut - more resources
Full Text Sources

