Developmental Windows for Effects of Choline and Folate on Excitatory and Inhibitory Neurotransmission During Human Gestation
- PMID: 38646069
- PMCID: PMC11031125
- DOI: 10.1002/dev.22453
Developmental Windows for Effects of Choline and Folate on Excitatory and Inhibitory Neurotransmission During Human Gestation
Abstract
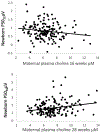
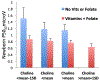
Choline and folate are critical nutrients for fetal brain development, but the timing of their influence during gestation has not been previously characterized. At different periods during gestation, choline stimulation of α7-nicotinic receptors facilitates conversion of γ-aminobutyric acid (GABA) receptors from excitatory to inhibitory and recruitment of GluR1-R2 receptors for faster excitatory responses to glutamate. The outcome of the fetal development of inhibition and excitation was assessed in 159 newborns by P50 cerebral auditory-evoked responses. Paired stimuli, S1, S2, were presented 500 msec apart. Higher P50 amplitude in response to S1 (P50S1microV) assesses excitation, and lower P50S2microV assesses inhibition in this paired-stimulus paradigm. Development of inhibition was related solely to maternal choline plasma concentration and folate supplementation at 16 weeks' gestation. Development of excitation was related only to maternal choline at 28 weeks. Higher maternal choline concentrations later in gestation did not compensate for earlier lower concentrations. At 4 years of age, increased behavior problems on the Child Behavior Checklist 1½-5yrs were related to both newborn inhibition and excitation. Incomplete development of inhibition and excitation associated with lower choline and folate during relatively brief periods of gestation thus has enduring effects on child development.
Keywords: Attention; Brain; Choline; Evoked Potentials Auditory; Excitation; Fetal Development; Hippocampus; Human; Inhibition; Phosphatidylcholine; Pregnancy.
Conflict of interest statement
Conflict of interest disclosure: Angelo D’Alessandro is a founder and Chief Scientific Officer of Omix Technologies, Inc. and Altis Biosciences, and Scientific Advisory Board member of Hemanext Inc. These companies were not involved in this study. The other authors declare no competing financial interests.
Figures
References
-
- Czeizel AE, Dudás I (1992) Prevention of the first occurrence of neural-tube defects by periconceptional vitamin supplementation N Engl J Med 327:1832–1835. - PubMed
-
- Cheatham CL, Goldman BD, Fischer LM, da Costa K-A, Reznick JS, Zeisel SH 2012. Phosphatidylcholine supplementation in pregnant women consuming moderate-choline diets does not enhance infant cognitive function: a randomized, double-blind, placebo-controlled trial. Am J Clin Nutrition 96:1465–1472. - PMC - PubMed
-
- Jacobson SW, Carter RC, Molteno CD, Stanton ME, Herbert JS, Lindinger NM, Lewis CE, Dodge NC, Hoyme HE, Zeisel SH, Meintjes EM, Duggan CP, Jacobson JL (2018) Efficacy of maternal choline supplementation during pregnancy in mitigating adverse effects of prenatal alcohol exposure on growth and cognitive function: A randomized, double-blind, placebo-controlled clinical trial Alcoholism: Clin Exp Res 42:1327–1341. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

