Focused stimulation of dorsal versus ventral subthalamic nucleus enhances action-outcome learning in patients with Parkinson's disease
- PMID: 38646144
- PMCID: PMC11032193
- DOI: 10.1093/braincomms/fcae111
Focused stimulation of dorsal versus ventral subthalamic nucleus enhances action-outcome learning in patients with Parkinson's disease
Abstract
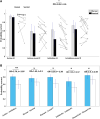
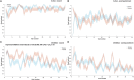
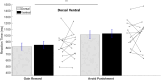
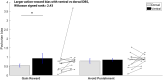
Deep brain stimulation of the subthalamic nucleus is an effective treatment for the clinical motor symptoms of Parkinson's disease, but may alter the ability to learn contingencies between stimuli, actions and outcomes. We investigated how stimulation of the functional subregions in the subthalamic nucleus (motor and cognitive regions) modulates stimulus-action-outcome learning in Parkinson's disease patients. Twelve Parkinson's disease patients with deep brain stimulation of the subthalamic nucleus completed a probabilistic stimulus-action-outcome task while undergoing ventral and dorsal subthalamic nucleus stimulation (within subjects, order counterbalanced). The task orthogonalized action choice and outcome valence, which created four action-outcome learning conditions: action-reward, inhibit-reward, action-punishment avoidance and inhibit-punishment avoidance. We compared the effects of deep brain stimulation on learning rates across these conditions as well as on computed Pavlovian learning biases. Dorsal stimulation was associated with higher overall learning proficiency relative to ventral subthalamic nucleus stimulation. Compared to ventral stimulation, stimulating the dorsal subthalamic nucleus led to a particular advantage in learning to inhibit action to produce desired outcomes (gain reward or avoid punishment) as well as better learning proficiency across all conditions providing reward opportunities. The Pavlovian reward bias was reduced with dorsal relative to ventral subthalamic nucleus stimulation, which was reflected by improved inhibit-reward learning. Our results show that focused stimulation in the dorsal compared to the ventral subthalamic nucleus is relatively more favourable for learning action-outcome contingencies and reduces the Pavlovian bias that could lead to reward-driven behaviour. Considering the effects of deep brain stimulation of the subthalamic nucleus on learning and behaviour could be important when optimizing stimulation parameters to avoid side effects like impulsive reward-driven behaviour.
Keywords: action–outcome learning; deep brain stimulation; subthalamic nucleus.
© The Author(s) 2024. Published by Oxford University Press on behalf of the Guarantors of Brain.
Conflict of interest statement
B.M.D. is a founder and equity holder in Neurotargeting, LLC, that licences the technology from Vanderbilt University described in this article.
Figures


References
-
- Ridderinkhof KR, Forstmann BU, Wylie SA, Burle B, van den Wildenberg WPM. Neurocognitive mechanisms of action control: Resisting the call of the Sirens. Wiley Interdiscip Rev Cogn Sci. 2011;2(2):174–192. - PubMed
-
- Hershberger WA. An approach through the looking-glass. Anim Learn Behav. 1986;14(4):443–451.
-
- DeLong MR, Wichmann T. Circuits and circuit disorders of the basal ganglia. Arch Neurol. 2007;64(1):20–24. - PubMed
-
- Dayan P, Niv Y, Seymour B, Daw ND. The misbehavior of value and the discipline of the will. Neural Netw. 2006;19(8):1153–1160. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
