Efficacy of Continuous Intravenous Fentanyl for Oral Mucosal Pain in Stevens-Johnson Syndrome: A Case Report
- PMID: 38646265
- PMCID: PMC11032730
- DOI: 10.7759/cureus.56735
Efficacy of Continuous Intravenous Fentanyl for Oral Mucosal Pain in Stevens-Johnson Syndrome: A Case Report
Abstract
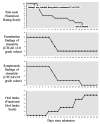
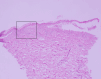
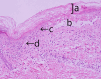
The management of oral mucosal pain in Stevens-Johnson syndrome (SJS), known for its severe mucocutaneous reactions, is a significant challenge due to the paucity of effective treatments reported in the literature. This case report aims to help fill this gap by describing the effective use of continuous intravenous fentanyl for the relief of severe oral mucosal pain in a patient with SJS. A patient with postoperative recurrence of cervical cancer developed SJS following chemotherapy. She had severe oral mucosal pain that was not relieved by 12.5 mcg/hour fentanyl transdermal patch, a regular medication. This pain was rated 10/10 on the Numerical Pain Rating Scale (NRS), and the patient had dysphagia and difficulty speaking. On admission, intravenous methylprednisolone (1000 mg/day), oral lip treatment with dexamethasone ointment, and oral rinses with azulene-lidocaine mixture were started. Analgesic treatment consisted of a 12.5 mcg/hour fentanyl transdermal patch of the regular medication and 1000 mg/dose of intravenous acetaminophen twice daily. Due to the inadequate efficacy of the transdermal patch, fentanyl was switched from the transdermal patch to a continuous intravenous fentanyl infusion at 20 mcg/hour on day three of admission. This adjustment significantly reduced pain intensity, which decreased to NRS 5/10 on day six of admission, and the patient was able to drink water and speak. Pain relief preceded clinical improvement of stomatitis. Grade 1 somnolence occurred after the start of intravenous fentanyl, but improved with follow-up. There were no other adverse effects such as respiratory depression. This case highlights the potential of intravenous fentanyl in the treatment of oral mucosal pain associated with SJS, although further studies are needed to confirm these findings and to develop comprehensive pain management protocols.
Keywords: fentanyl; morphine; oral mucosal lesions; stevens-johnson syndrome (sjs); stomatitis medicamentosa.
Copyright © 2024, Ito et al.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Similar articles
-
Patient-reported utilization patterns of fentanyl transdermal system and oxycodone hydrochloride controlled-release among patients with chronic nonmalignant pain.J Manag Care Pharm. 2003 May-Jun;9(3):223-31. doi: 10.18553/jmcp.2003.9.3.223. J Manag Care Pharm. 2003. PMID: 14613465 Free PMC article. Clinical Trial.
-
[Three-day-type transdermal fentanyl patch conversion by rapid titration method with short-acting oral oxycodone for cancer pain].Gan To Kagaku Ryoho. 2012 Mar;39(3):405-8. Gan To Kagaku Ryoho. 2012. PMID: 22421768 Clinical Trial. Japanese.
-
Transdermal fentanyl. A review of its pharmacological properties and therapeutic efficacy in pain control.Drugs. 1997 Jan;53(1):109-38. doi: 10.2165/00003495-199753010-00011. Drugs. 1997. PMID: 9010652 Review.
-
Clinical experience with transdermal and orally administered opioids in palliative care patients--a retrospective study.Jpn J Clin Oncol. 2007 Apr;37(4):302-9. doi: 10.1093/jjco/hym017. Epub 2007 May 22. Jpn J Clin Oncol. 2007. PMID: 17519302
-
Benefit-risk assessment of transdermal fentanyl for the treatment of chronic pain.Drug Saf. 2003;26(13):951-73. doi: 10.2165/00002018-200326130-00004. Drug Saf. 2003. PMID: 14583070 Review.
References
-
- U.K. guidelines for the management of Stevens-Johnson syndrome/toxic epidermal necrolysis in adults 2016. Creamer D, Walsh SA, Dziewulski P, et al. Br J Dermatol. 2016;174:1194–1227. - PubMed
-
- Nutrition in toxic epidermal necrolysis: a multicenter review. Graves C, Faraklas I, Maniatis K, et al. Nutr Clin Pract. 2016;31:836–840. - PubMed
-
- Post-traumatic stress disorder in Stevens-Johnson syndrome and toxic epidermal necrolysis: prevalence and risk factors. A prospective study of 31 patients. Hefez L, Zaghbib K, Sbidian E, et al. Br J Dermatol. 2019;180:1206–1213. - PubMed
-
- [Pain management in Stevens-Johnson syndrome, toxic epidermal necrolysis and other blistering diseases] Valeyrie-Allanore L, Ingen-Housz-Oro S, Colin A, Thuillot D, Sigal ML, Binhas M. Ann Dermatol Venereol. 2011;138:694–697. - PubMed
-
- A systematic review of the use of opioid medication for those with moderate to severe cancer pain and renal impairment: a European Palliative Care Research Collaborative opioid guidelines project. King S, Forbes K, Hanks GW, Ferro CJ, Chambers EJ. Palliat Med. 2011;25:525–552. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous
