Design, synthesis, and biochemical and computational screening of novel oxindole derivatives as inhibitors of Aurora A kinase and SARS-CoV-2 spike/host ACE2 interaction
- PMID: 38646411
- PMCID: PMC11024012
- DOI: 10.1007/s00044-024-03201-7
Design, synthesis, and biochemical and computational screening of novel oxindole derivatives as inhibitors of Aurora A kinase and SARS-CoV-2 spike/host ACE2 interaction
Abstract
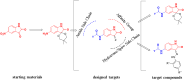
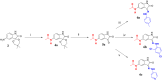
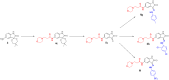
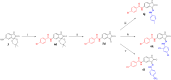
Isatin (indol-2,3-dione), a secondary metabolite of tryptophan, has been used as the core structure to design several compounds that have been tested and identified as potent inhibitors of apoptosis, potential antitumor agents, anticonvulsants, and antiviral agents. In this work, several analogs of isatin hybrids have been synthesized and characterized, and their activities were established as inhibitors of both Aurora A kinase and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike/host angiotensin-converting enzyme II (ACE2) interactions. Amongst the synthesized isatin hybrids, compounds 6a, 6f, 6g, and 6m exhibited Aurora A kinase inhibitory activities (with IC50 values < 5 M), with GScore values of -7.9, -7.6, -8.2 and -7.7 kcal/mol, respectively. Compounds 6g and 6i showed activities in blocking SARS-CoV-2 spike/ACE2 binding (with IC50 values in the range < 30 M), with GScore values of -6.4 and -6.6 kcal/mol, respectively. Compounds 6f, 6g, and 6i were both capable of inhibiting spike/ACE2 binding and blocking Aurora A kinase. Pharmacophore profiling indicated that compound 6g tightly fits Aurora A kinase and SARS-CoV-2 pharmacophores, while 6d fits SARS-CoV-2 and 6l fits Aurora A kinase pharmacophore. This work is a proof of concept that some existing cancer drugs may possess antiviral properties. Molecular modeling showed that the active compound for each protein adopted different binding modes, hence interacting with a different set of amino acid residues in the binding site. The weaker activities against spike/ACE2 could be explained by the small sizes of the ligands that fail to address the important interactions for binding to the ACE2 receptor site.
Keywords: ACE2; Aurora A kinase; SARS-CoV-2; spike/ACE2 interactions.
© This is a U.S. Government work and not under copyright protection in the US; foreign copyright protection may apply 2024.
Conflict of interest statement
Conflict of interestThe authors declare no competing interests.
Figures



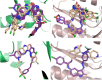

References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
