Incidence of drug-related adverse events related to the use of high-alert drugs: A systematic review of randomized controlled trials
- PMID: 38646469
- PMCID: PMC11031819
- DOI: 10.1016/j.rcsop.2024.100435
Incidence of drug-related adverse events related to the use of high-alert drugs: A systematic review of randomized controlled trials
Abstract
Background: High-alert medication (HAM) is more predictable to cause significant harm to the patient, even when used as intended. The damage related to the HAM lead not only suffering to the patient, but also raise the additional costs associated with care.
Objective: Evaluate the incidence of drug-related adverse events related to the use of high-alert medications.
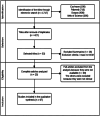
Methods: It was conducted an active search for information through COCHRANE databases, LILACS, SciELO, SCOPUS, PubMed/MEDLINE and WEB OF SCIENCE. The search strategy included the following terms: "Patient safety", "Medication errors" and "Hospital" and "High Alert Medications" or "Dangerous Drugs" in different combinations. Then two reviewers independently conducted a preliminary evaluation of relevant titles, abstracts and finally full-text. Studies quality was evaluated according to PRISMA declaration.
Results: The systematic review evaluated seven articles, which showed that only 11 HAM identified in the literature could have serious events. The most frequently cited were warfarin (22.2%) which progressed from deep vein thrombosis to gangrene, suggesting lower initial doses, followed by cyclophosphamide (22.2%) and cyclosporine (22.2%) which presented invasive fungal infection and death. In addition to these, morphine was compared with its active metabolite (M6G), with M6G causing fewer serious clinical events related to nausea and vomiting, reducing the need for concomitant use of antiemetics.
Conclusions: The most reported drug classes in the articles included that were related to incidence of drug-related adverse events in use of high-alert medications: morphine, M6G-glucuronide, haloperidol, promethazine, ivabradine, digoxin, warfarin, ximelagatran, cyclophosphamide, cyclosporine, and ATG. The formulate protocols for the use of these medications, with importance placed on evaluating, among the classes, the medication that causes the least harm.
Keywords: High-alert medication or dangerous drugs; Hospital; Medication errors; Patient safety.
© 2024 The Author(s).
Conflict of interest statement
None.
References
-
- WHO . 2006. World alliance for patient safety: forward programme 2006-2007.
Publication types
LinkOut - more resources
Full Text Sources
Research Materials