Immunoglobulins and serum proteins impair anti-tumor NK cell effector functions in malignant ascites
- PMID: 38646521
- PMCID: PMC11026578
- DOI: 10.3389/fimmu.2024.1360615
Immunoglobulins and serum proteins impair anti-tumor NK cell effector functions in malignant ascites
Erratum in
-
Corrigendum: Immunoglobulins and serum proteins impair anti-tumor NK cell effector functions in malignant ascites.Front Immunol. 2024 Apr 29;15:1420991. doi: 10.3389/fimmu.2024.1420991. eCollection 2024. Front Immunol. 2024. PMID: 38742114 Free PMC article.
Abstract
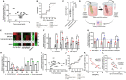
Introduction: Malignant ascites indicates ovarian cancer progression and predicts poor clinical outcome. Various ascites components induce an immunosuppressive crosstalk between tumor and immune cells, which is poorly understood. In our previous study, imbalanced electrolytes, particularly high sodium content in malignant ascites, have been identified as a main immunosuppressive mechanism that impaired NK and T-cell activity.
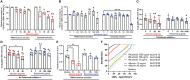
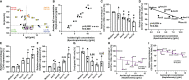
Methods: In the present study, we explored the role of high concentrations of ascites proteins and immunoglobulins on antitumoral NK effector functions. To this end, a coculture system consisting of healthy donor NK cells and ovarian cancer cells was used. The anti-EGFR antibody Cetuximab was added to induce antibody-dependent cellular cytotoxicity (ADCC). NK activity was assessed in the presence of different patient ascites samples and immunoglobulins that were isolated from ascites.
Results: Overall high protein concentration in ascites impaired NK cell degranulation, conjugation to tumor cells, and intracellular calcium signaling. Immunoglobulins isolated from ascites samples competitively interfered with NK ADCC and inhibited the conjugation to target cells. Furthermore, downregulation of regulatory surface markers CD16 and DNAM-1 on NK cells was prevented by ascites-derived immunoglobulins during NK cell activation.
Conclusion: Our data show that high protein concentrations in biological fluids are able to suppress antitumoral activity of NK cells independent from the mechanism mediated by imbalanced electrolytes. The competitive interference between immunoglobulins of ascites and specific therapeutic antibodies could diminish the efficacy of antibody-based therapies and should be considered in antibody-based immunotherapies.
Keywords: NK cells; albumin; antibody; ascites; immunoglobulins; immunosuppression; ovarian cancer; tumor microenvironment.
Copyright © 2024 Hrvat, Benders, Kimmig, Brandau and Mallmann-Gottschalk.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures

Similar articles
-
Electrolyte imbalance causes suppression of NK and T cell effector function in malignant ascites.J Exp Clin Cancer Res. 2023 Sep 8;42(1):235. doi: 10.1186/s13046-023-02798-8. J Exp Clin Cancer Res. 2023. PMID: 37684704 Free PMC article.
-
Monocytes and the 38kDa-antigen of mycobacterium tuberculosis modulate natural killer cell activity and their cytolysis directed against ovarian cancer cell lines.BMC Cancer. 2012 Oct 4;12:451. doi: 10.1186/1471-2407-12-451. BMC Cancer. 2012. PMID: 23036052 Free PMC article.
-
Natural Killer Cells Response to IL-2 Stimulation Is Distinct between Ascites with the Presence or Absence of Malignant Cells in Ovarian Cancer Patients.Int J Mol Sci. 2017 May 17;18(5):856. doi: 10.3390/ijms18050856. Int J Mol Sci. 2017. PMID: 28513532 Free PMC article.
-
ADCC: the rock band led by therapeutic antibodies, tumor and immune cells.Front Immunol. 2025 Apr 16;16:1548292. doi: 10.3389/fimmu.2025.1548292. eCollection 2025. Front Immunol. 2025. PMID: 40308580 Free PMC article. Review.
-
Antibody-Dependent Cell-Mediated Cytotoxicity Through Natural Killer (NK) Cells: Unlocking NK Cells for Future Immunotherapy.Curr Pharm Biotechnol. 2022;23(4):552-578. doi: 10.2174/1389201022666210820093608. Curr Pharm Biotechnol. 2022. PMID: 34414871 Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

