Mucosal immunization with a low-energy electron inactivated respiratory syncytial virus vaccine protects mice without Th2 immune bias
- PMID: 38646538
- PMCID: PMC11026718
- DOI: 10.3389/fimmu.2024.1382318
Mucosal immunization with a low-energy electron inactivated respiratory syncytial virus vaccine protects mice without Th2 immune bias
Abstract
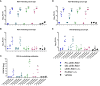
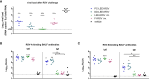
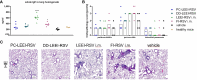
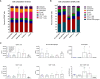
The respiratory syncytial virus (RSV) is a leading cause of acute lower respiratory tract infections associated with numerous hospitalizations. Recently, intramuscular (i.m.) vaccines against RSV have been approved for elderly and pregnant women. Noninvasive mucosal vaccination, e.g., by inhalation, offers an alternative against respiratory pathogens like RSV. Effective mucosal vaccines induce local immune responses, potentially resulting in the efficient and fast elimination of respiratory viruses after natural infection. To investigate this immune response to an RSV challenge, low-energy electron inactivated RSV (LEEI-RSV) was formulated with phosphatidylcholine-liposomes (PC-LEEI-RSV) or 1,2-dioleoyl-3-trimethylammonium-propane and 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine (DD-LEEI-RSV) for vaccination of mice intranasally. As controls, LEEI-RSV and formalin-inactivated-RSV (FI-RSV) were used via i.m. vaccination. The RSV-specific immunogenicity of the different vaccines and their protective efficacy were analyzed. RSV-specific IgA antibodies and a statistically significant reduction in viral load upon challenge were detected in mucosal DD-LEEI-RSV-vaccinated animals. Alhydrogel-adjuvanted LEEI-RSV i.m. showed a Th2-bias with enhanced IgE, eosinophils, and lung histopathology comparable to FI-RSV. These effects were absent when applying the mucosal vaccines highlighting the potential of DD-LEEI-RSV as an RSV vaccine candidate and the improved performance of this mucosal vaccine candidate.
Keywords: Respiratory Syncytial Virus (RSV); formulation; low-energy electron irradiation (LEEI); mucosal immunity; mucosal vaccination.
Copyright © 2024 Eberlein, Rosencrantz, Finkensieper, Besecke, Mansuroglu, Kamp, Lange, Dressman, Schopf, Hesse, Thoma, Fertey, Ulbert and Grunwald.
Conflict of interest statement
SU and MT are authors of patents and patent applications covering LEEI to inactivate liquids: WO2018041953, DE102015224206B3, US10080795, DE 102017002645.9. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
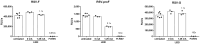

Similar articles
-
Mucosal Application of a Low-Energy Electron Inactivated Respiratory Syncytial Virus Vaccine Shows Protective Efficacy in an Animal Model.Viruses. 2023 Aug 30;15(9):1846. doi: 10.3390/v15091846. Viruses. 2023. PMID: 37766253 Free PMC article.
-
Immunization with an adjuvanted low-energy electron irradiation inactivated respiratory syncytial virus vaccine shows immunoprotective activity in mice.Vaccine. 2018 Mar 14;36(12):1561-1569. doi: 10.1016/j.vaccine.2018.02.014. Epub 2018 Feb 10. Vaccine. 2018. PMID: 29439869
-
Intranasal immunization with W 80 5EC adjuvanted recombinant RSV rF-ptn enhances clearance of respiratory syncytial virus in a mouse model.Hum Vaccin Immunother. 2014;10(3):615-22. doi: 10.4161/hv.27383. Epub 2013 Dec 10. Hum Vaccin Immunother. 2014. PMID: 24326268 Free PMC article.
-
Mucosal vaccines against respiratory syncytial virus.Curr Opin Virol. 2014 Jun;6:78-84. doi: 10.1016/j.coviro.2014.03.009. Epub 2014 Apr 29. Curr Opin Virol. 2014. PMID: 24794644 Free PMC article. Review.
-
Respiratory syncytial virus in infants: is maternal vaccination a realistic strategy?Curr Opin Infect Dis. 2015 Jun;28(3):221-4. doi: 10.1097/QCO.0000000000000161. Curr Opin Infect Dis. 2015. PMID: 25918956 Review.
Cited by
-
Novel Administration Routes, Delivery Vectors, and Application of Vaccines Based on Biotechnologies: A Review.Vaccines (Basel). 2024 Sep 1;12(9):1002. doi: 10.3390/vaccines12091002. Vaccines (Basel). 2024. PMID: 39340032 Free PMC article. Review.
-
Recent advances in the prevention and treatment of respiratory syncytial virus disease.J Gen Virol. 2025 Apr;106(4):002095. doi: 10.1099/jgv.0.002095. J Gen Virol. 2025. PMID: 40202895 Free PMC article. Review.
-
Evaluation of dual pathogen recognition receptor agonists as adjuvants for respiratory syncytial virus - virus-like particles for pulmonary delivery.Front Immunol. 2025 Mar 17;16:1561297. doi: 10.3389/fimmu.2025.1561297. eCollection 2025. Front Immunol. 2025. PMID: 40176816 Free PMC article.
References
-
- Li Y, Wang X, Blau DM, Caballero MT, Feikin DR, Gill CJ, et al. . Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in children younger than 5 years in 2019: a systematic analysis. Lancet. (2022) 399:2047–64. doi: 10.1016/S0140-6736(22)00478-0 - DOI - PMC - PubMed
-
- GSK . US FDA approves GSK’s Arexvy, the world’s first respiratory syncytial virus (RSV) vaccine for older adults | GSK (2023). Available at: https://www.gsk.com/en-gb/media/press-releases/us-fda-approves-gsk-s-are....
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

