Mucosal immunization with a low-energy electron inactivated respiratory syncytial virus vaccine protects mice without Th2 immune bias
- PMID: 38646538
- PMCID: PMC11026718
- DOI: 10.3389/fimmu.2024.1382318
Mucosal immunization with a low-energy electron inactivated respiratory syncytial virus vaccine protects mice without Th2 immune bias
Abstract
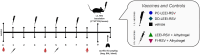
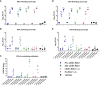
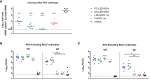
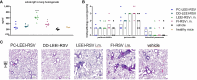
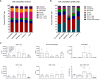
The respiratory syncytial virus (RSV) is a leading cause of acute lower respiratory tract infections associated with numerous hospitalizations. Recently, intramuscular (i.m.) vaccines against RSV have been approved for elderly and pregnant women. Noninvasive mucosal vaccination, e.g., by inhalation, offers an alternative against respiratory pathogens like RSV. Effective mucosal vaccines induce local immune responses, potentially resulting in the efficient and fast elimination of respiratory viruses after natural infection. To investigate this immune response to an RSV challenge, low-energy electron inactivated RSV (LEEI-RSV) was formulated with phosphatidylcholine-liposomes (PC-LEEI-RSV) or 1,2-dioleoyl-3-trimethylammonium-propane and 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine (DD-LEEI-RSV) for vaccination of mice intranasally. As controls, LEEI-RSV and formalin-inactivated-RSV (FI-RSV) were used via i.m. vaccination. The RSV-specific immunogenicity of the different vaccines and their protective efficacy were analyzed. RSV-specific IgA antibodies and a statistically significant reduction in viral load upon challenge were detected in mucosal DD-LEEI-RSV-vaccinated animals. Alhydrogel-adjuvanted LEEI-RSV i.m. showed a Th2-bias with enhanced IgE, eosinophils, and lung histopathology comparable to FI-RSV. These effects were absent when applying the mucosal vaccines highlighting the potential of DD-LEEI-RSV as an RSV vaccine candidate and the improved performance of this mucosal vaccine candidate.
Keywords: Respiratory Syncytial Virus (RSV); formulation; low-energy electron irradiation (LEEI); mucosal immunity; mucosal vaccination.
Copyright © 2024 Eberlein, Rosencrantz, Finkensieper, Besecke, Mansuroglu, Kamp, Lange, Dressman, Schopf, Hesse, Thoma, Fertey, Ulbert and Grunwald.
Conflict of interest statement
SU and MT are authors of patents and patent applications covering LEEI to inactivate liquids: WO2018041953, DE102015224206B3, US10080795, DE 102017002645.9. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
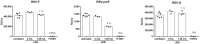

References
-
- Li Y, Wang X, Blau DM, Caballero MT, Feikin DR, Gill CJ, et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in children younger than 5 years in 2019: a systematic analysis. Lancet. (2022) 399:2047–64. doi: 10.1016/S0140-6736(22)00478-0 - DOI - PMC - PubMed
-
- GSK . US FDA approves GSK’s Arexvy, the world’s first respiratory syncytial virus (RSV) vaccine for older adults | GSK (2023). Available at: https://www.gsk.com/en-gb/media/press-releases/us-fda-approves-gsk-s-are....
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

