Pectin methylesterification state and cell wall mechanical properties contribute to neighbor proximity-induced hypocotyl growth in Arabidopsis
- PMID: 38646567
- PMCID: PMC11033045
- DOI: 10.1002/pld3.584
Pectin methylesterification state and cell wall mechanical properties contribute to neighbor proximity-induced hypocotyl growth in Arabidopsis
Abstract
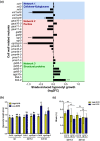
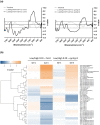
Plants growing with neighbors compete for light and consequently increase the growth of their vegetative organs to enhance access to sunlight. This response, called shade avoidance syndrome (SAS), involves photoreceptors such as phytochromes as well as phytochrome interacting factors (PIFs), which regulate the expression of growth-mediating genes. Numerous cell wall-related genes belong to the putative targets of PIFs, and the importance of cell wall modifications for enabling growth was extensively shown in developmental models such as dark-grown hypocotyl. However, the contribution of the cell wall in the growth of de-etiolated seedlings regulated by shade cues remains poorly established. Through analyses of mechanical and biochemical properties of the cell wall coupled with transcriptomic analysis of cell wall-related genes from previously published data, we provide evidence suggesting that cell wall modifications are important for neighbor proximity-induced elongation. Further analysis using loss-of-function mutants impaired in the synthesis and remodeling of the main cell wall polymers corroborated this. We focused on the cgr2cgr3 double mutant that is defective in methylesterification of homogalacturonan (HG)-type pectins. By following hypocotyl growth kinetically and spatially and analyzing the mechanical and biochemical properties of cell walls, we found that methylesterification of HG-type pectins was required to enable global cell wall modifications underlying neighbor proximity-induced hypocotyl growth. Collectively, our work suggests that plant competition for light induces changes in the expression of numerous cell wall genes to enable modifications in biochemical and mechanical properties of cell walls that contribute to neighbor proximity-induced growth.
© 2024 The Authors. Plant Direct published by American Society of Plant Biologists and the Society for Experimental Biology and John Wiley & Sons Ltd.
Conflict of interest statement
The authors did not report any conflict of interest.
Figures



References
-
- Alonso‐simón, A. , García‐angulo, P. , Mélida, H. , Encina, A. , Álvarez, J. M. , & Acebes, J. L. (2011). The use of FTIR spectroscopy to monitor modifications in plant cell wall architecture caused by cellulose biosynthesis inhibitors. Plant Signaling & Behavior, 6, 1104–1110. 10.4161/psb.6.8.15793 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

