Trem2 promotes foamy macrophage lipid uptake and survival in atherosclerosis
- PMID: 38646596
- PMCID: PMC11031198
- DOI: 10.1038/s44161-023-00354-3
Trem2 promotes foamy macrophage lipid uptake and survival in atherosclerosis
Abstract
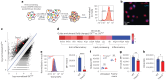
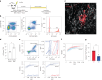
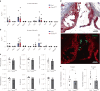
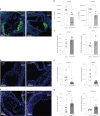
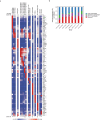
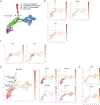
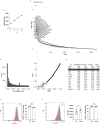
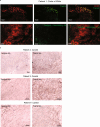
Atherosclerosis is driven by the expansion of cholesterol-loaded 'foamy' macrophages in the arterial intima. Factors regulating foamy macrophage differentiation and survival in plaque remain poorly understood. Here we show, using trajectory analysis of integrated single-cell RNA sequencing data and a genome-wide CRISPR screen, that triggering receptor expressed on myeloid cells 2 (Trem2) is associated with foamy macrophage specification. Loss of Trem2 led to a reduced ability of foamy macrophages to take up oxidized low-density lipoprotein (oxLDL). Myeloid-specific deletion of Trem2 showed an attenuation of plaque progression, even when targeted in established atherosclerotic lesions, and was independent of changes in circulating cytokines, monocyte recruitment or cholesterol levels. Mechanistically, we link Trem2-deficient macrophages with a failure to upregulate cholesterol efflux molecules, resulting in impaired proliferation and survival. Overall, we identify Trem2 as a regulator of foamy macrophage differentiation and atherosclerotic plaque growth and as a putative therapeutic target for atherosclerosis.
Conflict of interest statement
Competing interests The authors declare no competing interests.
Figures








References
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
