Disturbed flow impairs MerTK-mediated efferocytosis in aortic endothelial cells during atherosclerosis
- PMID: 38646649
- PMCID: PMC11024847
- DOI: 10.7150/thno.93036
Disturbed flow impairs MerTK-mediated efferocytosis in aortic endothelial cells during atherosclerosis
Abstract
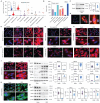
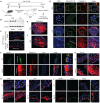
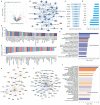
Background: MER proto-oncogene tyrosine kinase (MerTK) is a key receptor for efferocytosis, a process for the clearance of apoptotic cells. MerTK is mainly expressed in macrophages and immature dendritic cells. There are very limited reports focused on MerTK biology in aortic endothelial cells (ECs). It remains unclear for the role of blood flow patterns in regulating MerTK-mediated efferocytosis in aortic ECs. This study was designed to investigate whether endothelial MerTK and EC efferocytosis respond to blood flow patterns during atherosclerosis. Methods: Big data analytics, RNA-seq and proteomics combined with our in vitro and in vivo studies were applied to reveal the potential molecular mechanisms. Partial carotid artery ligation combined with AAV-PCSK9 and high fat diet were used to set up acute atherosclerosis in 4 weeks. Results: Our data showed that MerTK is sensitive to blood flow patterns and is inhibited by disturbed flow and oscillatory shear stress in primary human aortic ECs (HAECs). The RNA-seq data in HAECs incubated with apoptotic cells showed that d-flow promotes pro-inflammatory pathway and senescence pathway. Our in vivo data of proteomics and immunostaining showed that, compared with WT group, MerTK-/- aggravates atherosclerosis in d-flow areas through upregulation of endothelial dysfunction markers (e.g. IL-1β, NF-κB, TLR4, MAPK signaling, vWF, VCAM-1 and p22phox) and mitochondrial dysfunction. Interestingly, MerTK-/-induces obvious abnormal endothelial thickening accompanied with decreased endothelial efferocytosis, promoting the development of atherosclerosis. Conclusions: Our data suggests that blood flow patterns play an important role in regulating MerTK-mediated efferocytosis in aortic ECs, revealing a new promising therapeutic strategy with EC efferocytosis restoration to against atherosclerosis.
Keywords: Disturbed flow; MerTK; RNA-seq; atherosclerosis; efferocytosis; proteomics.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures



References
-
- Herrington W, Lacey B, Sherliker P, Armitage J, Lewington S. Epidemiology of atherosclerosis and the potential to reduce the global burden of atherothrombotic disease. Circ Res. 2016;118:535–46. - PubMed
-
- Endo S, Goldsmith H L, Karino T. Flow patterns and preferred sites of atherosclerotic lesions in the human aorta-II. Abdominal aorta. Biorheology. 2014;51:257–274. - PubMed
-
- Ando J, Yamamoto K. Flow detection and calcium signaling in vascular endothelial cells. Cardiovasc Res. 2013;99:260–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

