Involvement of kinesins in skeletal dysplasia: a review
- PMID: 38646780
- PMCID: PMC11293425
- DOI: 10.1152/ajpcell.00613.2023
Involvement of kinesins in skeletal dysplasia: a review
Abstract
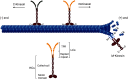
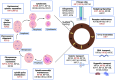
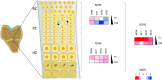
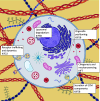
Skeletal dysplasias are group of rare genetic diseases resulting from mutations in genes encoding structural proteins of the cartilage extracellular matrix (ECM), signaling molecules, transcription factors, epigenetic modifiers, and several intracellular proteins. Cell division, organelle maintenance, and intracellular transport are all orchestrated by the cytoskeleton-associated proteins, and intracellular processes affected through microtubule-associated movement are important for the function of skeletal cells. Among microtubule-associated motor proteins, kinesins in particular have been shown to play a key role in cell cycle dynamics, including chromosome segregation, mitotic spindle formation, and ciliogenesis, in addition to cargo trafficking, receptor recycling, and endocytosis. Recent studies highlight the fundamental role of kinesins in embryonic development and morphogenesis and have shown that mutations in kinesin genes lead to several skeletal dysplasias. However, many questions concerning the specific functions of kinesins and their adaptor molecules as well as specific molecular mechanisms in which the kinesin proteins are involved during skeletal development remain unanswered. Here we present a review of the skeletal dysplasias resulting from defects in kinesins and discuss the involvement of kinesin proteins in the molecular mechanisms that are active during skeletal development.
Keywords: chondrocyte; kinesin; microtubules; motor proteins; skeletal dysplasia.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures

References
-
- Warman ML, Cormier‐Daire V, Hall C, Krakow D, Lachman R, Lemerrer M, Mortier G, Mundlos S, Nishimura G, Rimoin DL, Robertson S, Savarirayan R, Sillence D, Spranger J, Unger S, Zabel B, Superti‐Furga A. Nosology and classification of genetic skeletal disorders: 2010 revision. Am J Med Genet A 155A: 943–968, 2011. doi:10.1002/ajmg.a.33909. - DOI - PMC - PubMed
-
- Unger S, Ferreira CR, Mortier GR, Ali H, Bertola DR, Calder A, Cohn DH, Cormier-Daire V, Girisha KM, Hall C, Krakow D, Makitie O, Mundlos S, Nishimura G, Robertson SP, Savarirayan R, Sillence D, Simon M, Sutton VR, Warman ML, Superti-Furga A. Nosology of genetic skeletal disorders: 2023 revision. Am J Med Genet A 191: 1164–1209, 2023. doi:10.1002/ajmg.a.63132. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

