Japan-Multimodal Intervention Trial for the Prevention of Dementia: A randomized controlled trial
- PMID: 38646854
- PMCID: PMC11180858
- DOI: 10.1002/alz.13838
Japan-Multimodal Intervention Trial for the Prevention of Dementia: A randomized controlled trial
Abstract
Introduction: We examined the efficacy of a multidomain intervention in preventing cognitive decline among Japanese older adults with mild cognitive impairment (MCI).
Methods: Participants aged 65-85 years with MCI were randomized into intervention (management of vascular risk factors, exercise, nutritional counseling, and cognitive training) and control groups. The primary outcome was changes in the cognitive composite score over a period of 18 months.
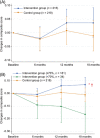
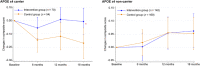
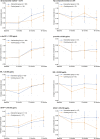
Results: Of 531 participants, 406 completed the trial. The between-group difference in composite score changes was 0.047 (95% CI: -0.029 to 0.124). Secondary analyses indicated positive impacts of interventions on several secondary health outcomes. The interventions appeared to be particularly effective for individuals with high attendance during exercise sessions and those with the apolipoprotein E ε4 allele and elevated plasma glial fibrillary acidic protein levels.
Discussion: The multidomain intervention showed no efficacy in preventing cognitive decline. Further research on more efficient strategies and suitable target populations is required.
Highlights: This trial evaluated the efficacy of multidomain intervention in individuals with MCI. The trial did not show a significant difference in preplanned cognitive outcomes. Interventions had positive effects on a wide range of secondary health outcomes. Those with adequate adherence or high risk of dementia benefited from interventions.
Keywords: cognitive decline; cognitive training; dementia; diet; mild cognitive impairment; multidomain intervention; nutrition; physical exercise; randomized control trial.
© 2024 The Authors. Alzheimer's & Dementia published by Wiley Periodicals LLC on behalf of Alzheimer's Association.
Conflict of interest statement
National Center for Geriatrics and Gerontology (NCGG), the employer of Akinori Nakamura, has filed and registered the following patents: AU 2016322342, AU 2019205010, CN 201680053338.0, EP 16846400.6, EP 19192797.9, IN 201847013746, JP 6467512, and US 15/752498. Akinori Nakamura is the inventor of these patents, but all patent rights have been transferred to NCGG. All other authors declare no competing interests. Author disclosures are available in the Supporting information.
Figures

References
-
- Prince M, Wimo A, Guerchet M, Ali GC, Wu YT, Prina M. World Alzheimer Report 2015 – The Global Impact of Dementia: Alzheimer's Disease International; 2015.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

