Pyrimidine depletion enhances targeted and immune therapy combinations in acute myeloid leukemia
- PMID: 38646934
- PMCID: PMC11141866
- DOI: 10.1172/jci.insight.173646
Pyrimidine depletion enhances targeted and immune therapy combinations in acute myeloid leukemia
Abstract
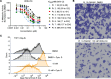
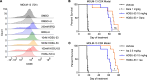
Acute myeloid leukemia (AML) is a fatal disease characterized by the accumulation of undifferentiated myeloblasts, and agents that promote differentiation have been effective in this disease but are not curative. Dihydroorotate dehydrogenase inhibitors (DHODHi) have the ability to promote AML differentiation and target aberrant malignant myelopoiesis. We introduce HOSU-53, a DHODHi with significant monotherapy activity, which is further enhanced when combined with other standard-of-care therapeutics. We further discovered that DHODHi modulated surface expression of CD38 and CD47, prompting the evaluation of HOSU-53 combined with anti-CD38 and anti-CD47 therapies, where we identified a compelling curative potential in an aggressive AML model with CD47 targeting. Finally, we explored using plasma dihydroorotate (DHO) levels to monitor HOSU-53 safety and found that the level of DHO accumulation could predict HOSU-53 intolerability, suggesting the clinical use of plasma DHO to determine safe DHODHi doses. Collectively, our data support the clinical translation of HOSU-53 in AML, particularly to augment immune therapies. Potent DHODHi to date have been limited by their therapeutic index; however, we introduce pharmacodynamic monitoring to predict tolerability while preserving antitumor activity. We additionally suggest that DHODHi is effective at lower doses with select immune therapies, widening the therapeutic index.
Keywords: Cancer immunotherapy; Drug therapy; Leukemias; Oncology; Therapeutics.
Figures






References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

