In utero and postnatal ivacaftor/lumacaftor therapy rescues multiorgan disease in CFTR-F508del ferrets
- PMID: 38646935
- PMCID: PMC11141870
- DOI: 10.1172/jci.insight.157229
In utero and postnatal ivacaftor/lumacaftor therapy rescues multiorgan disease in CFTR-F508del ferrets
Abstract
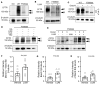
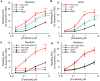
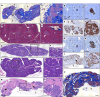
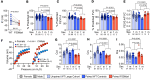
Cystic fibrosis (CF) is caused by mutations in the CF transmembrane conductance regulator (CFTR) gene, with F508del being the most prevalent mutation. The combination of CFTR modulators (potentiator and correctors) has provided benefit to CF patients carrying the F508del mutation; however, the safety and effectiveness of in utero combination modulator therapy remains unclear. We created a F508del ferret model to test whether ivacaftor/lumacaftor (VX-770/VX-809) therapy can rescue in utero and postnatal pathologies associated with CF. Using primary intestinal organoids and air-liquid interface cultures of airway epithelia, we demonstrate that the F508del mutation in ferret CFTR results in a severe folding and trafficking defect, which can be partially restored by treatment with CFTR modulators. In utero treatment of pregnant jills with ivacaftor/lumacaftor prevented meconium ileus at birth in F508del kits and sustained postnatal treatment of CF offspring improved survival and partially protected from pancreatic insufficiency. Withdrawal of ivacaftor/lumacaftor treatment from juvenile CF ferrets reestablished pancreatic and lung diseases, with altered pulmonary mechanics. These findings suggest that in utero intervention with a combination of CFTR modulators may provide therapeutic benefits to individuals with F508del. This CFTR-F508del ferret model may be useful for testing therapies using clinically translatable endpoints.
Keywords: Bacterial infections; Genetic diseases; Pulmonology; Therapeutics.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

