Venous vascular closure system vs. figure-of-eight suture following atrial fibrillation ablation: the STYLE-AF Study
- PMID: 38647070
- PMCID: PMC11210072
- DOI: 10.1093/europace/euae105
Venous vascular closure system vs. figure-of-eight suture following atrial fibrillation ablation: the STYLE-AF Study
Abstract
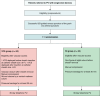
Aims: Simplified ablation technologies for pulmonary vein isolation (PVI) are increasingly performed worldwide. One of the most common complications following PVI are vascular access-related complications. Lately, venous closure systems (VCSs) were introduced into clinical practice, aiming to reduce the time of bed rest, to increase the patients' comfort, and to reduce vascular access-related complications. The aim of the present study is to compare the safety and efficacy of using a VCS to achieve haemostasis following single-shot PVI to the actual standard of care [figure-of-eight suture and manual compression (MC)].
Methods and results: This is a prospective, multicentre, randomized, controlled, open-label trial performed at three German centres. Patients were randomized 1:1 to undergo haemostasis either by means of VCS (VCS group) or of a figure-of-eight suture and MC (F8 group). The primary efficacy endpoint was the time to ambulation, while the primary safety endpoint was the incidence of major periprocedural adverse events until hospital discharge. A total of 125 patients were randomized. The baseline characteristics were similar between the groups. The VCS group showed a shorter time to ambulation [109.0 (82.0, 160.0) vs. 269.0 (243.8, 340.5) min; P < 0.001], shorter time to haemostasis [1 (1, 2) vs. 5 (2, 10) min; P < 0.001], and shorter time to discharge eligibility [270 (270, 270) vs. 340 (300, 458) min; P < 0.001]. No major vascular access-related complication was reported in either group. A trend towards a lower incidence of minor vascular access-related complications on the day of procedure was observed in the VCS group [7 (11.1%) vs. 15 (24.2%); P = 0.063] as compared to the control group.
Conclusion: Following AF ablation, the use of a VCS results in a significantly shorter time to ambulation, time to haemostasis, and time to discharge eligibility. No major vascular access-related complications were identified. The use of MC and a figure-of-eight suture showed a trend towards a higher incidence of minor vascular access-related complications.
Keywords: Atrial fibrillation; Complications; Cryoballoon; Pulse field ablation; Vascular closure device.
© The Author(s) 2024. Published by Oxford University Press on behalf of the European Society of Cardiology.
Conflict of interest statement
Conflict of interest: R.R.T. is a consultant for Boston Scientific, Biotronik, Biosense Webster, and Abbott Medical, and he received speaker’s honoraria from Boston Scientific, Biotronik, Biosense Webster, Abbott Medical, and Lifetech and research grants from Abbott, Biosense Webster, and Lifetech. C.-H.H. received travel grants and research grants from Abbott Medical, Boston Scientific, Medtronic, Lifetech, Biosense Webster, and CardioFocus. He is a consultant for Boston Scientific, Biosense Webster, and Lifetech and received speaker honoraria from Abbott Medical, Boston Scientific, Medtronic, Biosense Webster, CardioFocus, Pfizer, BMS, C.T.I. GmbH, and Doctrina Med. K.-H.K. reports grants and personal fees from Abbott Vascular, Medtronic, and Biosense Webster outside the submitted work. C.E. received travel grants and research grants from Abbott, Boston Scientific, and Biosense Webster and speaker honoraria from Abbott, Boston Scientific, Biosense Webster, C.T.I. GmbH, and Doctrina Med. S.S.P. received travel grants and congress grants from Lifetech and an Educational Grant and speaker honoraria from Abbott Medical. J.V. received honoraria for lectures / talks: Abbott, Astra Zeneca, Pfizer, Boston Scientific, C.T.I., Doctrina Med, Medtronic and travel grants: Medtronic, Boston Scientific, Abbott, and is proctor for Boston Scientific. P.S. is Member of Advisory Board of Abbott, Biosense Webster, Boston Scientific and Medtronic. All other authors have no relevant disclosures.
Figures
References
-
- Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström-Lundqvist C, et al. . 2020 esc guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J 2021;42:373–498. - PubMed
-
- Kuck K-H, Brugada J, Fürnkranz A, Metzner A, Ouyang F, Chun KRJ, et al. . Cryoballoon or radiofrequency ablation for paroxysmal atrial fibrillation. N Engl J Med 2016;374:2235–45. - PubMed
-
- Reddy VY, Gerstenfeld EP, Natale A, Whang W, Cuoco FA, Patel C, et al. . Pulsed field or conventional thermal ablation for paroxysmal atrial fibrillation. N Engl J Med 2023;389:1660–71. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous