Controlling Candida: immune regulation of commensal fungi in the gut
- PMID: 38647290
- PMCID: PMC11385159
- DOI: 10.1128/iai.00516-23
Controlling Candida: immune regulation of commensal fungi in the gut
Abstract
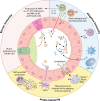
The intestinal microbiome harbors fungi that pose a significant risk to human health as opportunistic pathogens and drivers of inflammation. Inflammatory and autoimmune diseases are associated with dysbiotic fungal communities and the expansion of potentially pathogenic fungi. The gut is also the main reservoir for disseminated fungal infections. Immune interactions are critical for preventing commensal fungi from becoming pathogenic. Significant strides have been made in defining innate and adaptive immune pathways that regulate intestinal fungi, and these discoveries have coincided with advancements in our understanding of the fungal molecular pathways and effectors involved in both commensal colonization and pathogenesis within the gut. In this review, we will discuss immune interactions important for regulating commensal fungi, with a focus on how specific cell types and effectors interact with fungi to limit their colonization or pathogenic potential. This will include how innate and adaptive immune pathways target fungi and orchestrate antifungal immune responses, in addition to how secreted immune effectors, such as mucus and antimicrobial peptides, regulate fungal colonization and inhibit pathogenic potential. These immune interactions will be framed around our current understanding of the fungal effectors and pathways regulating colonization and pathogenesis within this niche. Finally, we highlight important unexplored mechanisms by which the immune system regulates commensal fungi in the gut.
Keywords: Candida; Candida albicans; gut microbiota; microbiome; mucosal immunity; mycobiome.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Limon JJ, Tang J, Li D, Wolf AJ, Michelsen KS, Funari V, Gargus M, Nguyen C, Sharma P, Maymi VI, Iliev ID, Skalski JH, Brown J, Landers C, Borneman J, Braun J, Targan SR, McGovern DPB, Underhill DM. 2019. Malassezia is associated with Crohn’s disease and exacerbates colitis in mouse models. Cell Host Microbe 25:377–388. doi:10.1016/j.chom.2019.01.007 - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

