Hybrid Membrane-Coated Nanoparticles for Precise Targeting and Synergistic Therapy in Alzheimer's Disease
- PMID: 38647399
- PMCID: PMC11200089
- DOI: 10.1002/advs.202306675
Hybrid Membrane-Coated Nanoparticles for Precise Targeting and Synergistic Therapy in Alzheimer's Disease
Abstract
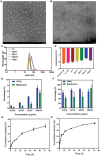
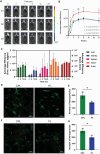
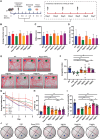
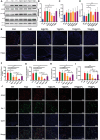
The blood brain barrier (BBB) limits the application of most therapeutic drugs for neurological diseases (NDs). Hybrid cell membrane-coated nanoparticles derived from different cell types can mimic the surface properties and functionalities of the source cells, further enhancing their targeting precision and therapeutic efficacy. Neuroinflammation has been increasingly recognized as a critical factor in the pathogenesis of various NDs, especially Alzheimer's disease (AD). In this study, a novel cell membrane coating is designed by hybridizing the membrane from platelets and chemokine (C-C motif) receptor 2 (CCR2) cells are overexpressed to cross the BBB and target neuroinflammatory lesions. Past unsuccessful endeavors in AD drug development underscore the challenge of achieving favorable outcomes when utilizing single-mechanism drugs.Two drugs with different mechanisms of actions into liposomes are successfully loaded to realize multitargeting treatment. In a transgenic mouse model for familial AD (5xFAD), the administration of these drug-loaded hybrid cell membrane liposomes results in a significant reduction in amyloid plaque deposition, neuroinflammation, and cognitive impairments. Collectively, the hybrid cell membrane-coated nanomaterials offer new opportunities for precise drug delivery and disease-specific targeting, which represent a versatile platform for targeted therapy in AD.
Keywords: Alzheimer's disease; hybrid cell membrane‐coated liposomes; inflammatory targeting; synergistic therapy.
© 2024 The Authors. Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
MeSH terms
Substances
Grants and funding
- 2021ZD0201103/Science Innovation 2030-Brain Science and Brain-Inspired Intelligence Technology Major Projects
- 2021ZD0201803/Science Innovation 2030-Brain Science and Brain-Inspired Intelligence Technology Major Projects
- 2024SSYS0018/Key R&D Program of Zhejiang Province
- LBY21H090003/Natural Science Foundation of Zhejiang Province
- 2022ZDYF22/Key Science & Technologies R&D Program of Lishui City
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
