Effectiveness of the online-based comprehensive cognitive training application, Smart Brain, for community-dwelling older adults with dementia: a randomized controlled trial
- PMID: 38647533
- PMCID: PMC11261305
- DOI: 10.23736/S1973-9087.24.08043-2
Effectiveness of the online-based comprehensive cognitive training application, Smart Brain, for community-dwelling older adults with dementia: a randomized controlled trial
Abstract
Background: The fourth industrial revolution has brought about developments in information and communication technologies for interventions in older adults with dementia. Currently, most interventions focus on single interventions. However, community-dwelling older adults with dementia require comprehensive cognitive interventions, and clinical studies analyzing the effects of comprehensive interventions based on randomized controlled trials are lacking.
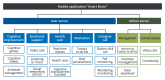
Aim: The aim of the study was to examine the effects of an information and communication technology-based comprehensive cognitive training program, Smart Brain, on multi-domain function among community-dwelling older adults with dementia.
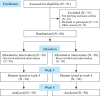
Design: This was a two-group, randomized, controlled trial.
Setting: This study was conducted at participant's home.
Population: We analyzed older adults with dementia.
Methods: Participants were randomly allocated to either the intervention group (N.=30) or the control group (N.=30). Older adults with dementia in the intervention group received 8 weeks of Smart Brain comprehensive cognitive training using a tablet, whereas the control group received a similar tablet but without the training. We measured the outcomes at baseline, and at 4 and 8 weeks. Cognitive function, depression, quality of life, balance confidence, physical ability, nutrition, and caregiver burden were compared between groups.
Results: In the intervention group, cognitive function statistically increased from baseline to both week 4 (2.03; 95% CI 1.26 to 2.81) and week 8 (2.70; 95% CI 1.76 to 3.64). Depression was statistically different from week 0 to week 8 (-1.67, 95% CI -2.85 to -0.48). Physical ability statistically increased from baseline to both week 4 (-0.85; 95% CI 1.49 to -0.20) and week 8 (-1.44; 95% CI -2.29 to -0.59). Nutrition statistically increased from baseline to both week 4 (0.67; 95% CI 0.05 to 1.28) and week 8 (1.10; 95% CI 0.36 to 1.84).
Conclusions: Smart Brain significantly improved cognitive function, reduced depression, and enhanced physical and nutritional status in older adults with dementia. This demonstrates its potential as an effective non-pharmacological intervention in community-based dementia care.
Clinical rehabilitation impact: Smart Brain's personalized approach, which integrates user-specific preferences and expert guidance, enhances engagement and goal achievement in dementia care. This enhances self-esteem and clinical outcomes, demonstrates the application's potential to innovate rehabilitation practices.
Conflict of interest statement
Figures
Similar articles
-
Feasibility of a person-centred multidimensional interdisciplinary rehabilitation programme in community-dwelling people with dementia: a randomised controlled pilot trial.BMC Geriatr. 2024 Sep 28;24(1):794. doi: 10.1186/s12877-024-05372-9. BMC Geriatr. 2024. PMID: 39342131 Free PMC article. Clinical Trial.
-
Behavioural interventions for urinary incontinence in community-dwelling seniors: an evidence-based analysis.Ont Health Technol Assess Ser. 2008;8(3):1-52. Epub 2008 Oct 1. Ont Health Technol Assess Ser. 2008. PMID: 23074508 Free PMC article.
-
Efficacy of an Intelligent and Integrated Older Adult Care Model on Quality of Life Among Home-Dwelling Older Adults: Randomized Controlled Trial.J Med Internet Res. 2025 Apr 21;27:e67950. doi: 10.2196/67950. J Med Internet Res. 2025. PMID: 40258267 Free PMC article. Clinical Trial.
-
The effectiveness of e-interventions on fall, neuromuscular functions and quality of life in community-dwelling older adults: A systematic review and meta-analysis.Int J Nurs Stud. 2021 Jan;113:103784. doi: 10.1016/j.ijnurstu.2020.103784. Epub 2020 Oct 3. Int J Nurs Stud. 2021. PMID: 33120138
-
The effectiveness of smart home technologies to support the health outcomes of community-dwelling older adults living with dementia: A scoping review.Int J Med Inform. 2021 Sep;153:104513. doi: 10.1016/j.ijmedinf.2021.104513. Epub 2021 Jun 4. Int J Med Inform. 2021. PMID: 34116363
Cited by
-
Effectiveness, Adoption Determinants, and Implementation Challenges of ICT-Based Cognitive Support for Older Adults with MCI and Dementia: A PRISMA-Compliant Systematic Review and Meta-Analysis (2015-2025).Healthcare (Basel). 2025 Jun 13;13(12):1421. doi: 10.3390/healthcare13121421. Healthcare (Basel). 2025. PMID: 40565449 Free PMC article. Review.
References
-
- World Health Organization. Dementia; 2021 [Internet]. Available from: https://www.who.int/news-room/fact-sheets/detail/dementia1 [cited 2024, Apr 8].
-
- Mielke MM, Leoutsakos JM, Corcoran CD, Green RC, Norton MC, Welsh-Bohmer KA, et al. Effects of Food and Drug Administration-approved medications for Alzheimer’s disease on clinical progression. Alzheimers Dement 2012;8:180–7. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&l... 10.1016/j.jalz.2011.02.011 - DOI - PMC - PubMed
-
- Epperly T, Dunay MA, Boice JL. Alzheimer Disease: Pharmacologic and Nonpharmacologic Therapies for Cognitive and Functional Symptoms. Am Fam Physician 2017;95:771–8. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&l... - PubMed
-
- Dyer SM, Harrison SL, Laver K, Whitehead C, Crotty M. An overview of systematic reviews of pharmacological and non-pharmacological interventions for the treatment of behavioral and psychological symptoms of dementia. Int Psychogeriatr 2018;30:295–309. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&l... 10.1017/S1041610217002344 - DOI - PubMed
-
- Lissek V, Suchan B. Preventing dementia? Interventional approaches in mild cognitive impairment. Neurosci Biobehav Rev 2021;122:143–64. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&l... 10.1016/j.neubiorev.2020.12.022 - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical